Dolutegravir-lamivudine as initial therapy in HIV-1 infected, ARV-naive patients, 48-week results of the PADDLE (Pilot Antiretroviral Design with Dolutegravir LamivudinE) study
- PMID: 28537061
- PMCID: PMC5515053
- DOI: 10.7448/IAS.20.01.21678
Dolutegravir-lamivudine as initial therapy in HIV-1 infected, ARV-naive patients, 48-week results of the PADDLE (Pilot Antiretroviral Design with Dolutegravir LamivudinE) study
Abstract
Introduction: A proof-of-concept study was designed to evaluate the antiviral efficacy, safety and tolerability of a two-drug regimen with dolutegravir 50 mg once daily (QD) plus lamivudine 300 mg once daily as initial highly active antiretroviral therapy (HAART) among antiretroviral (ARV)-naive patients.
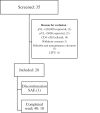
Methods: PADDLE is a pilot study including 20 treatment-naive adults. To be selected, participants had no IAS-USA-defined resistance, HIV-1 RNA ≤100,000 copies/mL at screening and negative HBsAg. Plasma viral load (pVL) was measured at baseline; days 2, 4, 7, 10, 14, 21 and 28; weeks 6, 8 and 12; and thereafter every 12 weeks up to 96 weeks. Primary endpoint was the proportion of patients with HIV-1 RNA <50 copies/mL in an intention to treat (ITT)-exposed analysis at 48 weeks (the FDA snapshot algorithm).
Results: Median HIV-1 RNA at entry was 24,128 copies/mL (interquartile range (IQR): 11,686-36,794). Albeit as per protocol, all patients had pVL ≤100,000 copies/mL at screening as required by inclusion criteria, four patients had ≥100,000 copies/mL at baseline. Median baseline CD4+ T-cell count was 507 per cubic millimetre (IQR: 296-517). A rapid decline in pVL was observed (median VL decay from baseline to week 12 was 2.74 logs). All patients were suppressed at week 8 onwards up to week 24. At week 48, 90% (18/20) reached the primary endpoint of a pVL <50 copies/mL. Median change in CD4 cell count between baseline and week 48 was 267 cells/mm3 (IQR: 180-462). No major tolerability/toxicity issues were observed. Nineteen patients completed 48 weeks of the study, and one patient (with undetectable VL at last visit) committed suicide. One patient presented a low-level protocol-defined confirmed virological failure at week 36, being the only observed failure. This patient had pVL <50 copies/mL at the end-of-study visit without having changed the two-drug regimen. Observed failure rate was 5%. This is the first report of integrase strand transfer inhibitor/lamivudine dual regimen in ARV-naive patients.
Conclusions: This novel dual regimen of dolutegravir and lamivudine warrants further clinical research and consideration as a potential therapeutic option for ARV-therapy-naive patients.
Clinicaltrials.gov identifier: NCT02211482.
Keywords: dolutegravir; dual therapy; lamivudine; naive patients.
Figures
References
-
- Gulick RM, Mellors JW, Havlir D, Eron JJ, Gonzalez C, McMahon D, et al. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med. 1997;337:734–7. - PubMed
-
- Montaner JS, Reiss P, Cooper D, Vella S, Harris M, Conway B, et al. A randomized, double-blind trial comparing combinations of nevirapine, didanosine, and zidovudine for HIV-infected patients: the INCAS trial. Italy, The Netherlands, Canada and Australia Study. JAMA. 1998;279:930–37. [cited 2016 January10] Available from: http://www.ncbi.nlm.nih.gov/pubmed/9544767 - PubMed
-
- UNAIDS AIDS by the numbers 2015; 2015.
-
- Cihlar T, Fordyce M.. Current status and prospects of HIV treatment. Curr Opin Virol. 2016;18:50–6. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials