Antigen-specific CD8+ T cell feedback activates NLRP3 inflammasome in antigen-presenting cells through perforin
- PMID: 28537251
- PMCID: PMC5458103
- DOI: 10.1038/ncomms15402
Antigen-specific CD8+ T cell feedback activates NLRP3 inflammasome in antigen-presenting cells through perforin
Abstract
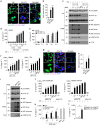
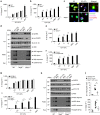
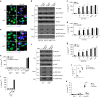
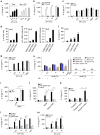
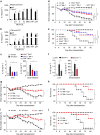
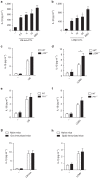
The connection between innate and adaptive immunity is best exemplified by antigen presentation. Although antigen-presenting cells (APCs) are required for antigen receptor-mediated T-cell activation, how T-cells feedback to APCs to sustain an antigen-specific immune response is not completely clear. Here we show that CD8+ T-cell (also called cytotoxic T lymphocytes, CTL) feedback activates the NLRP3 inflammasome in APCs in an antigen-dependent manner to promote IL-1β maturation. Perforin from antigen-specific CTLs is required for NLRP3 inflammasome activation in APCs. Furthermore, such activation of NLRP3 inflammasome contributes to the induction of antigen-specific antitumour immunity and pathogenesis of graft-versus-host diseases. Our study reveals a positive feedback loop between antigen-specific CTLs and APC to amplify adaptive immunity.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

Similar articles
-
The AIM2 and NLRP3 inflammasomes trigger IL-1-mediated antitumor effects during radiation.Sci Immunol. 2021 May 7;6(59):eabc6998. doi: 10.1126/sciimmunol.abc6998. Sci Immunol. 2021. PMID: 33963060
-
Nlrp3-dependent IL-1β inhibits CD103+ dendritic cell differentiation in the gut.JCI Insight. 2018 Mar 8;3(5):e96322. doi: 10.1172/jci.insight.96322. JCI Insight. 2018. PMID: 29515025 Free PMC article.
-
NOD-Like Receptor Protein 3 Inflammasome-Dependent IL-1β Accelerated ConA-Induced Hepatitis.Front Immunol. 2018 Apr 10;9:758. doi: 10.3389/fimmu.2018.00758. eCollection 2018. Front Immunol. 2018. PMID: 29692782 Free PMC article.
-
The NLRP3 inflammasome in kidney disease and autoimmunity.Nephrology (Carlton). 2016 Sep;21(9):736-44. doi: 10.1111/nep.12785. Nephrology (Carlton). 2016. PMID: 27011059 Review.
-
NLRP3 Inflammasome: Checkpoint Connecting Innate and Adaptive Immunity in Autoimmune Diseases.Front Immunol. 2021 Oct 11;12:732933. doi: 10.3389/fimmu.2021.732933. eCollection 2021. Front Immunol. 2021. PMID: 34707607 Free PMC article. Review.
Cited by
-
Ankylosing spondylitis: an autoimmune or autoinflammatory disease?Nat Rev Rheumatol. 2021 Jul;17(7):387-404. doi: 10.1038/s41584-021-00625-y. Epub 2021 Jun 10. Nat Rev Rheumatol. 2021. PMID: 34113018 Review.
-
Inflammasomes: potential therapeutic targets in hematopoietic stem cell transplantation.Cell Commun Signal. 2024 Dec 18;22(1):596. doi: 10.1186/s12964-024-01974-3. Cell Commun Signal. 2024. PMID: 39695742 Free PMC article. Review.
-
USP38 critically promotes asthmatic pathogenesis by stabilizing JunB protein.J Exp Med. 2018 Nov 5;215(11):2850-2867. doi: 10.1084/jem.20172026. Epub 2018 Sep 17. J Exp Med. 2018. PMID: 30224386 Free PMC article.
-
Immunopathogenesis of Immune Checkpoint Inhibitor-Related Adverse Events: Roles of the Intestinal Microbiome and Th17 Cells.Front Immunol. 2019 Sep 26;10:2254. doi: 10.3389/fimmu.2019.02254. eCollection 2019. Front Immunol. 2019. PMID: 31616428 Free PMC article. Review.
-
Multiple Sclerosis patients carry an increased burden of exceedingly rare genetic variants in the inflammasome regulatory genes.Sci Rep. 2019 Jun 24;9(1):9171. doi: 10.1038/s41598-019-45598-x. Sci Rep. 2019. PMID: 31235738 Free PMC article.
References
-
- Lamkanfi M. & Dixit V. M. Mechanisms and functions of inflammasomes. Cell 157, 1013–1022 (2014). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

