Evolutionary dynamics and genomic features of the Elizabethkingia anophelis 2015 to 2016 Wisconsin outbreak strain
- PMID: 28537263
- PMCID: PMC5458099
- DOI: 10.1038/ncomms15483
Evolutionary dynamics and genomic features of the Elizabethkingia anophelis 2015 to 2016 Wisconsin outbreak strain
Abstract
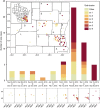
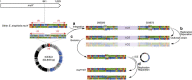
An atypically large outbreak of Elizabethkingia anophelis infections occurred in Wisconsin. Here we show that it was caused by a single strain with thirteen characteristic genomic regions. Strikingly, the outbreak isolates show an accelerated evolutionary rate and an atypical mutational spectrum. Six phylogenetic sub-clusters with distinctive temporal and geographic dynamics are revealed, and their last common ancestor existed approximately one year before the first recognized human infection. Unlike other E. anophelis, the outbreak strain had a disrupted DNA repair mutY gene caused by insertion of an integrative and conjugative element. This genomic change probably contributed to the high evolutionary rate of the outbreak strain and may have increased its adaptability, as many mutations in protein-coding genes occurred during the outbreak. This unique discovery of an outbreak caused by a naturally occurring mutator bacterial pathogen provides a dramatic example of the potential impact of pathogen evolutionary dynamics on infectious disease epidemiology.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



Comment in
-
Clinical Profile and Outcome of Neonates with Elizabethkingia Sepsis.Indian J Pediatr. 2023 Jun;90(6):612-614. doi: 10.1007/s12098-022-04467-8. Epub 2023 Jan 30. Indian J Pediatr. 2023. PMID: 36715863
References
-
- Kampfer P. et al. Elizabethkingia anophelis sp. nov., isolated from the midgut of the mosquito Anopheles gambiae. Int. J. Syst. Evol. Microbiol. 61, 2670–2675 (2011). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

