Aspirin-Mediated Acetylation Protects Against Multiple Neurodegenerative Pathologies by Impeding Protein Aggregation
- PMID: 28537433
- PMCID: PMC5661865
- DOI: 10.1089/ars.2016.6978
Aspirin-Mediated Acetylation Protects Against Multiple Neurodegenerative Pathologies by Impeding Protein Aggregation
Abstract
Aims: Many progressive neurological disorders, including Alzheimer's disease (AD), Huntington's disease, and Parkinson's disease (PD), are characterized by accumulation of insoluble protein aggregates. In prospective trials, the cyclooxygenase inhibitor aspirin (acetylsalicylic acid) reduced the risk of AD and PD, as well as cardiovascular events and many late-onset cancers. Considering the role played by protein hyperphosphorylation in aggregation and neurodegenerative diseases, and aspirin's known ability to donate acetyl groups, we asked whether aspirin might reduce both phosphorylation and aggregation by acetylating protein targets.
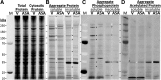
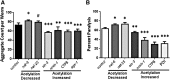
Results: Aspirin was substantially more effective than salicylate in reducing or delaying aggregation in human neuroblastoma cells grown in vitro, and in Caenorhabditis elegans models of human neurodegenerative diseases in vivo. Aspirin acetylates many proteins, while reducing phosphorylation, suggesting that acetylation may oppose phosphorylation. Surprisingly, acetylated proteins were largely excluded from compact aggregates. Molecular-dynamic simulations indicate that acetylation of amyloid peptide energetically disfavors its association into dimers and octamers, and oligomers that do form are less compact and stable than those comprising unacetylated peptides.
Innovation: Hyperphosphorylation predisposes certain proteins to aggregate (e.g., tau, α-synuclein, and transactive response DNA-binding protein 43 [TDP-43]), and it is a critical pathogenic marker in both cardiovascular and neurodegenerative diseases. We present novel evidence that acetylated proteins are underrepresented in protein aggregates, and that aggregation varies inversely with acetylation propensity after diverse genetic and pharmacologic interventions.
Conclusions: These results are consistent with the hypothesis that aspirin inhibits protein aggregation and the ensuing toxicity of aggregates through its acetyl-donating activity. This mechanism may contribute to the neuro-protective, cardio-protective, and life-prolonging effects of aspirin. Antioxid. Redox Signal. 27, 1383-1396.
Keywords: (protein) acetylation; (protein) aggregation; (protein) phosphorylation; aspirin (acetylsalicylic acid); inflammation; neurodegeneration.
Conflict of interest statement
No competing financial interests exist.
Figures






Similar articles
-
Proteins in aggregates functionally impact multiple neurodegenerative disease models by forming proteasome-blocking complexes.Aging Cell. 2015 Feb;14(1):35-48. doi: 10.1111/acel.12296. Epub 2014 Dec 16. Aging Cell. 2015. PMID: 25510159 Free PMC article.
-
Aspirin-mediated acetylation induces structural alteration and aggregation of bovine pancreatic insulin.J Biomol Struct Dyn. 2016;34(2):362-75. doi: 10.1080/07391102.2015.1039584. Epub 2015 Jun 23. J Biomol Struct Dyn. 2016. PMID: 25994118
-
Phthalocyanines as Molecular Scaffolds to Block Disease-Associated Protein Aggregation.Acc Chem Res. 2016 May 17;49(5):801-8. doi: 10.1021/acs.accounts.5b00507. Epub 2016 May 2. Acc Chem Res. 2016. PMID: 27136297 Review.
-
Proteins that mediate protein aggregation and cytotoxicity distinguish Alzheimer's hippocampus from normal controls.Aging Cell. 2016 Oct;15(5):924-39. doi: 10.1111/acel.12501. Epub 2016 Jul 23. Aging Cell. 2016. PMID: 27448508 Free PMC article.
-
Targeting oligomers in neurodegenerative disorders: lessons from α-synuclein, tau, and amyloid-β peptide.J Alzheimers Dis. 2011;24 Suppl 2:223-32. doi: 10.3233/JAD-2011-110182. J Alzheimers Dis. 2011. PMID: 21460436 Review.
Cited by
-
A universal transcriptomic signature of age reveals the temporal scaling of Caenorhabditis elegans aging trajectories.Sci Rep. 2019 May 14;9(1):7368. doi: 10.1038/s41598-019-43075-z. Sci Rep. 2019. PMID: 31089188 Free PMC article.
-
Aspirin inhibits proteasomal degradation and promotes α-synuclein aggregate clearance through K63 ubiquitination.Nat Commun. 2025 Feb 7;16(1):1438. doi: 10.1038/s41467-025-56737-6. Nat Commun. 2025. PMID: 39920137 Free PMC article.
-
A Novel Microtubule-Binding Drug Attenuates and Reverses Protein Aggregation in Animal Models of Alzheimer's Disease.Front Mol Neurosci. 2019 Dec 12;12:310. doi: 10.3389/fnmol.2019.00310. eCollection 2019. Front Mol Neurosci. 2019. PMID: 31920540 Free PMC article.
-
Thiadiazolidinone (TDZD) Analogs Inhibit Aggregation-Mediated Pathology in Diverse Neurodegeneration Models, and Extend C. elegans Life- and Healthspan.Pharmaceuticals (Basel). 2023 Oct 20;16(10):1498. doi: 10.3390/ph16101498. Pharmaceuticals (Basel). 2023. PMID: 37895969 Free PMC article.
-
Clinical Applications of Aspirin as a Multi-potent Drug Beyond Cardiovascular Implications: A Proof of Concept for Anesthesiologists- A Narrative Review.Anesth Pain Med. 2021 Oct 31;11(5):e118909. doi: 10.5812/aapm.118909. eCollection 2021 Oct. Anesth Pain Med. 2021. PMID: 35075415 Free PMC article. Review.
References
-
- Alfonso LF, Srivenugopal KS, and Bhat GJ. Does aspirin acetylate multiple cellular proteins? (Review). Mol Med Rep 2:533–537, 2009 - PubMed
-
- Ayyadevara S, Bharill P, Dandapat A, et al. . Aspirin inhibits oxidant stress, reduces age-associated functional declines, and extends lifespan of Caenorhabditis elegans. Antioxid Redox Signal 18: 481–490, 2013 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
