Capturing Human Naïve Pluripotency in the Embryo and in the Dish
- PMID: 28537488
- PMCID: PMC5564037
- DOI: 10.1089/scd.2017.0055
Capturing Human Naïve Pluripotency in the Embryo and in the Dish
Abstract
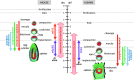
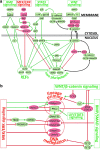
Although human embryonic stem cells (hESCs) were first derived almost 20 years ago, it was only recently acknowledged that they share closer molecular and functional identity to postimplantation lineage-primed murine epiblast stem cells than to naïve preimplantation inner cell mass-derived mouse ESCs (mESCs). A myriad of transcriptional, epigenetic, biochemical, and metabolic attributes have now been described that distinguish naïve and primed pluripotent states in both rodents and humans. Conventional hESCs and human induced pluripotent stem cells (hiPSCs) appear to lack many of the defining hallmarks of naïve mESCs. These include important features of the naïve ground state murine epiblast, such as an open epigenetic architecture, reduced lineage-primed gene expression, and chimera and germline competence following injection into a recipient blastocyst-stage embryo. Several transgenic and chemical methods were recently reported that appear to revert conventional human PSCs to mESC-like ground states. However, it remains unclear if subtle deviations in global transcription, cell signaling dependencies, and extent of epigenetic/metabolic shifts in these various human naïve-reverted pluripotent states represent true functional differences or alternatively the existence of distinct human pluripotent states along a spectrum. In this study, we review the current understanding and developmental features of various human pluripotency-associated phenotypes and discuss potential biological mechanisms that may support stable maintenance of an authentic epiblast-like ground state of human pluripotency.
Keywords: blastocyst; epiblast; hESC; human embryonic stem cell; inner cell mass; naive human pluripotency.
Conflict of interest statement
No competing financial interests exist.
Figures


Similar articles
-
Contrasting transcriptome landscapes of rabbit pluripotent stem cells in vitro and in vivo.Anim Reprod Sci. 2014 Sep;149(1-2):67-79. doi: 10.1016/j.anireprosci.2014.05.014. Epub 2014 Jul 1. Anim Reprod Sci. 2014. PMID: 25059199
-
The post-inner cell mass intermediate: implications for stem cell biology and assisted reproductive technology.Hum Reprod Update. 2015 Sep-Oct;21(5):616-26. doi: 10.1093/humupd/dmv028. Epub 2015 Jun 18. Hum Reprod Update. 2015. PMID: 26089403 Review.
-
Derivation of novel human ground state naive pluripotent stem cells.Nature. 2013 Dec 12;504(7479):282-6. doi: 10.1038/nature12745. Epub 2013 Oct 30. Nature. 2013. PMID: 24172903
-
Highly Efficient Derivation of Pluripotent Stem Cells from Mouse Preimplantation and Postimplantation Embryos in Serum-Free Conditions.Methods Mol Biol. 2019;2005:29-36. doi: 10.1007/978-1-4939-9524-0_2. Methods Mol Biol. 2019. PMID: 31175643
-
Pluripotent states of human embryonic stem cells.Cell Reprogram. 2015 Feb;17(1):1-6. doi: 10.1089/cell.2014.0061. Epub 2014 Nov 13. Cell Reprogram. 2015. PMID: 25393391 Free PMC article. Review.
Cited by
-
MicroRNAs and Stem-like Properties: The Complex Regulation Underlying Stemness Maintenance and Cancer Development.Biomolecules. 2021 Jul 21;11(8):1074. doi: 10.3390/biom11081074. Biomolecules. 2021. PMID: 34439740 Free PMC article. Review.
-
A single cell-based computational platform to identify chemical compounds targeting desired sets of transcription factors for cellular conversion.Stem Cell Reports. 2023 Jan 10;18(1):131-144. doi: 10.1016/j.stemcr.2022.10.013. Epub 2022 Nov 17. Stem Cell Reports. 2023. PMID: 36400030 Free PMC article.
-
Adipose Stem Cell Translational Applications: From Bench-to-Bedside.Int J Mol Sci. 2018 Nov 5;19(11):3475. doi: 10.3390/ijms19113475. Int J Mol Sci. 2018. PMID: 30400641 Free PMC article. Review.
-
Generation of Pericytic-Vascular Progenitors from Tankyrase/PARP-Inhibitor-Regulated Naïve (TIRN) Human Pluripotent Stem Cells.Methods Mol Biol. 2022;2416:133-156. doi: 10.1007/978-1-0716-1908-7_10. Methods Mol Biol. 2022. PMID: 34870835 Free PMC article.
-
Chemical Reversion of Conventional Human Pluripotent Stem Cells to a Naïve-like State with Improved Multilineage Differentiation Potency.J Vis Exp. 2018 Jun 10;(136):57921. doi: 10.3791/57921. J Vis Exp. 2018. PMID: 29939183 Free PMC article.
References
-
- Driesch H. (1894). Analytische Theorie der Organischen Entwicklung. W. Engelmann, Leipzig
-
- Nicholas JS. and Hall B. (1942). Experiments on developing rats. II. The development of isolated blastomeres and fused eggs. J Exp Zool 90:441–459
-
- Sheng G. (2015). Epiblast morphogenesis before gastrulation. Dev Biol 401:17–24 - PubMed
-
- Suwinska A, Czolowska R, Ozdzenski W. and Tarkowski AK. (2008). Blastomeres of the mouse embryo lose totipotency after the fifth cleavage division: expression of Cdx2 and Oct4 and developmental potential of inner and outer blastomeres of 16- and 32-cell embryos. Dev Biol 322:133–144 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

