Zidovudine use in pregnancy and congenital malformations
- PMID: 28537936
- PMCID: PMC5534355
- DOI: 10.1097/QAD.0000000000001549
Zidovudine use in pregnancy and congenital malformations
Abstract
Objective: There is inconsistent evidence that zidovudine use during pregnancy increases overall, cardiac, and male genital malformations.
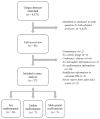
Design: We conducted a systematic review and meta-analysis of zidovudine use and malformations and, using Bayesian methods, combined it with data from a cohort study of mother-infant pairs in the nationwide Medicaid Analytic eXtract (MAX).
Methods: Using MAX data (2000-2010), we identified pregnant women with HIV treated with antiretroviral therapy (ART). Women with at least one zidovudine dispensing during the first trimester were compared to women receiving ART without zidovudine in the first trimester. Malformation outcomes were defined using diagnosis/procedure codes. To adjust for confounding, we performed 1 : 1 propensity score matching. Bayesian methods require specification of a prior, which we developed in the meta-analysis. Logistic regression models combined MAX data with the prior, estimating odds ratios (ORs) and 95% credible intervals.
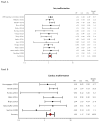
Results: Fourteen articles contributed information on overall malformations, seven on cardiac malformations, and five on male genital malformations. In MAX, matching led to a sample of 735 women each in the zidovudine and comparator groups. When comparing first trimester zidovudine use to other ART, the Bayesian procedure yielded OR estimates slightly above the null for overall [OR = 1.11, 95% credible interval (0.80-1.55)] and cardiac [OR = 1.30 (0.63-2.71)] malformations. There were no zidovudine-exposed cases of male genital malformations in MAX, but the meta-analysis yielded elevated OR estimates [OR = 2.57 (1.26-5.24)].
Conclusion: For most malformations, first-trimester zidovudine was not associated with increased risk. The potential increase in male genital malformations was small in absolute terms, and should be evaluated further.
Conflict of interest statement
KH is co-investigator of a grant to the Brigham and Women’s Hospital from Eli Lilly and from Pfizer, unrelated to the topic of this manuscript. BTB is co-investigator of a grant to the Brigham and Women’s Hospital or Massachusetts General Hospital from Eli Lilly, GSK, Pacira, Baxalta, and Pfizer. SHD received salary support from the North American AED Pregnancy Registry; and consulted for UCB, Teva, and Boehringer-Ingelheim; her institution received training grants from Pfizer, Takeda, Bayer, and Asisa. She is co-investigator grants to the Harvard T.H. Chan School of Public Health from Eli Lilly, GSK and Pfizer, unrelated to the topic of this manuscript.
Figures

References
-
- Cooper ER, Charurat M, Mofenson L, Hanson IC, Pitt J, Diaz C, et al. Combination antiretroviral strategies for the treatment of pregnant HIV-1-infected women and prevention of perinatal HIV-1 transmission. J Acquir Immune Defici Syndr. 2002;29:484–94. - PubMed
-
- World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. World Health Organization; 2016. http://www.who.int/hiv/pub/arv/arv-2016/en/ - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

