Genetic and Pharmacologic Inactivation of ANGPTL3 and Cardiovascular Disease
- PMID: 28538136
- PMCID: PMC5800308
- DOI: 10.1056/NEJMoa1612790
Genetic and Pharmacologic Inactivation of ANGPTL3 and Cardiovascular Disease
Abstract
Background: Loss-of-function variants in the angiopoietin-like 3 gene (ANGPTL3) have been associated with decreased plasma levels of triglycerides, low-density lipoprotein (LDL) cholesterol, and high-density lipoprotein (HDL) cholesterol. It is not known whether such variants or therapeutic antagonism of ANGPTL3 are associated with a reduced risk of atherosclerotic cardiovascular disease.
Methods: We sequenced the exons of ANGPTL3 in 58,335 participants in the DiscovEHR human genetics study. We performed tests of association for loss-of-function variants in ANGPTL3 with lipid levels and with coronary artery disease in 13,102 case patients and 40,430 controls from the DiscovEHR study, with follow-up studies involving 23,317 case patients and 107,166 controls from four population studies. We also tested the effects of a human monoclonal antibody, evinacumab, against Angptl3 in dyslipidemic mice and against ANGPTL3 in healthy human volunteers with elevated levels of triglycerides or LDL cholesterol.
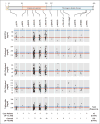
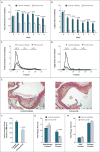
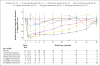
Results: In the DiscovEHR study, participants with heterozygous loss-of-function variants in ANGPTL3 had significantly lower serum levels of triglycerides, HDL cholesterol, and LDL cholesterol than participants without these variants. Loss-of-function variants were found in 0.33% of case patients with coronary artery disease and in 0.45% of controls (adjusted odds ratio, 0.59; 95% confidence interval, 0.41 to 0.85; P=0.004). These results were confirmed in the follow-up studies. In dyslipidemic mice, inhibition of Angptl3 with evinacumab resulted in a greater decrease in atherosclerotic lesion area and necrotic content than a control antibody. In humans, evinacumab caused a dose-dependent placebo-adjusted reduction in fasting triglyceride levels of up to 76% and LDL cholesterol levels of up to 23%.
Conclusions: Genetic and therapeutic antagonism of ANGPTL3 in humans and of Angptl3 in mice was associated with decreased levels of all three major lipid fractions and decreased odds of atherosclerotic cardiovascular disease. (Funded by Regeneron Pharmaceuticals and others; ClinicalTrials.gov number, NCT01749878 .).
Figures

Comment in
-
Dyslipidaemia: ANGPTL3: a therapeutic target for atherosclerosis.Nat Rev Cardiol. 2017 Jun 14;14(7):381. doi: 10.1038/nrcardio.2017.91. Nat Rev Cardiol. 2017. PMID: 28612836 No abstract available.
-
Cardiovascular endocrinology: Is ANGPTL3 the next PCSK9?Nat Rev Endocrinol. 2017 Sep;13(9):503-504. doi: 10.1038/nrendo.2017.88. Epub 2017 Jul 14. Nat Rev Endocrinol. 2017. PMID: 28707678 No abstract available.
-
Increasing Lipolysis and Reducing Atherosclerosis.N Engl J Med. 2017 Jul 20;377(3):280-283. doi: 10.1056/NEJMe1706907. N Engl J Med. 2017. PMID: 28723326 No abstract available.
-
Heart disease: Putative medicines that mimic mutations.Nature. 2017 Aug 31;548(7669):530-531. doi: 10.1038/nature23544. Epub 2017 Aug 23. Nature. 2017. PMID: 28847003 No abstract available.
References
-
- Wang H, Eckel RH. Lipoprotein lipase: from gene to obesity. Am J Physiol Endocrinol Metab. 2009;297:E271–E288. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
