Lanthanides Report Calcium Sensor in the Vestibule of Ryanodine Receptor
- PMID: 28538150
- PMCID: PMC5443974
- DOI: 10.1016/j.bpj.2017.03.023
Lanthanides Report Calcium Sensor in the Vestibule of Ryanodine Receptor
Abstract
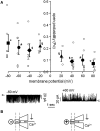
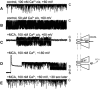
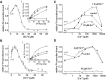
Ca2+ regulates ryanodine receptor's (RyR) activity through an activating and an inhibiting Ca2+-binding site located on the cytoplasmic side of the RyR channel. Their altered sensitivity plays an important role in the pathology of malignant hyperthermia and heart failure. We used lanthanide ions (Ln3+) as probes to investigate the Ca2+ sensors of RyR, because they specifically bind to Ca2+-binding proteins and they are impermeable to the channel. Eu3+'s and Sm3+'s action was tested on single RyR1 channels reconstituted into planar lipid bilayers. When the activating binding site was saturated by 50 μM Ca2+, Ln3+ potently inhibited RyR's open probability (Kd Eu3+ = 167 ± 5 nM and Kd Sm3+ = 63 ± 3 nM), but in nominally 0 [Ca2+], low [Eu3+] activated the channel. These results suggest that Ln3+ acts as an agonist of both Ca2+-binding sites. More importantly, the voltage-dependent characteristics of Ln3+'s action led to the conclusion that the activating Ca2+ binding site is located within the electrical field of the channel (in the vestibule). This idea was tested by applying the pore blocker toxin maurocalcine on the cytoplasmic side of RyR. These experiments showed that RyR lost reactivity to changing cytosolic [Ca2+] from 50 μM to 100 nM when the toxin occupied the vestibule. These results suggest that maurocalcine mechanically prevented Ca2+ from dissociating from its binding site and support our vestibular Ca2+ sensor-model further.
Copyright © 2017 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
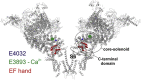



Similar articles
-
Eudistomin D and penaresin derivatives as modulators of ryanodine receptor channels and sarcoplasmic reticulum Ca2+ ATPase in striated muscle.Mol Pharmacol. 2014 Apr;85(4):564-75. doi: 10.1124/mol.113.089342. Epub 2014 Jan 14. Mol Pharmacol. 2014. PMID: 24423447 Free PMC article.
-
Redox-sensitive stimulation of type-1 ryanodine receptors by the scorpion toxin maurocalcine.Cell Calcium. 2013 May-Jun;53(5-6):357-65. doi: 10.1016/j.ceca.2013.03.004. Epub 2013 Apr 24. Cell Calcium. 2013. PMID: 23623374
-
Coupled calcium release channels and their regulation by luminal and cytosolic ions.Eur Biophys J. 2005 Jul;34(5):359-68. doi: 10.1007/s00249-005-0483-y. Epub 2005 May 25. Eur Biophys J. 2005. PMID: 15915341 Review.
-
Modification of ryanodine receptor/Ca2+ release channel with dinitrofluorobenzene.Biochem J. 1999 Aug 15;342 ( Pt 1)(Pt 1):239-48. doi: 10.1042/0264-6021:3420239. Biochem J. 1999. PMID: 10432322 Free PMC article.
-
Ca2+ stores regulate ryanodine receptor Ca2+ release channels via luminal and cytosolic Ca2+ sites.Clin Exp Pharmacol Physiol. 2007 Sep;34(9):889-96. doi: 10.1111/j.1440-1681.2007.04708.x. Clin Exp Pharmacol Physiol. 2007. PMID: 17645636 Review.
Cited by
-
The structural basis of ryanodine receptor ion channel function.J Gen Physiol. 2017 Dec 4;149(12):1065-1089. doi: 10.1085/jgp.201711878. Epub 2017 Nov 9. J Gen Physiol. 2017. PMID: 29122978 Free PMC article. Review.
-
Eu3+ detects two functionally distinct luminal Ca2+ binding sites in ryanodine receptors.Biophys J. 2023 Sep 5;122(17):3516-3531. doi: 10.1016/j.bpj.2023.07.029. Epub 2023 Aug 2. Biophys J. 2023. PMID: 37533257 Free PMC article.
-
Function of a mutant ryanodine receptor (T4709M) linked to congenital myopathy.Sci Rep. 2023 Sep 5;13(1):14659. doi: 10.1038/s41598-023-41801-2. Sci Rep. 2023. PMID: 37670077 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

