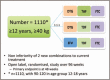
The ADVANCE study: a groundbreaking trial to evaluate a candidate universal antiretroviral regimen
- PMID: 28538284
- PMCID: PMC5459583
- DOI: 10.1097/COH.0000000000000389
The ADVANCE study: a groundbreaking trial to evaluate a candidate universal antiretroviral regimen
Abstract
Purpose of review: Current WHO-recommended first-line therapy in low-income and middle-income countries has been very successful in saving millions of lives but still has toxicity concerns and a low barrier to resistance.
Recent findings: Two candidate antiretrovirals may substantially transform first-line therapy in low-income and middle-income countries, yielding a safer, more robust and cheaper regimen. Tenofovir alafenamide carries toxicity and cost benefits over tenofovir disoproxil fumarate. Dolutegravir could replace efavirenz, with substantial toxicity, resistance and cost benefits. However, these drugs are currently not manufactured together in developed countries, for commercial reasons.
Summary: We describe a large randomized controlled study testing a combination of these candidate antiretrovirals against the current first-line recommendation that commenced recruitment in early 2017. We justify the study design and discuss how we will deal with complex issues such as tuberculosis and pregnancy.
Figures
References
-
- Raffi F, Pozniak AL, Wainberg MA. Has the time come to abandon efavirenz for first-line antiretroviral therapy? J Antimicrob Chemother 2014; 69:1742–1747. - PubMed
-
- Clinton Health Access Initiative. The state of the antiretroviral drug market in low-and middle-income countries, 2015–2020. Published October 21st, 2016. Available from: http://www.clintonhealthaccess.org/arv-mket-report-2016/. [Accessed 20 March 2017]
-
This invaluable resource documents the most recent data on costs and antiretroviral usage.
-
- WHO. Geneva. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Recommendations for a public health approach – Second edition. Published June 2016. Available from: http://www.who.int/hiv/pub/arv/arv-2016/en/. [Accessed 1 October 2016]
-
Low-income and middle-income countries use WHO antiretroviral guidelines to guide drug choices for national guidelines.
-
- I-Base. Fit for purpose: antiretroviral treatment optimisation (Feb 2017). London. Published Feb 2017. Available from: http://i-base.info/fit-for-purpose-feb-2017/. [Accessed 20 March 2017]
-
This guide categorizes all current critical antiretroviral treatment registration studies, as well as discussing new approached to diagnosing and treating HIV, hepatitis and tuberculosis.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials