Thalidomide results in diminished ovarian reserve in reproductive age female IBD patients
- PMID: 28538364
- PMCID: PMC5457844
- DOI: 10.1097/MD.0000000000006540
Thalidomide results in diminished ovarian reserve in reproductive age female IBD patients
Abstract
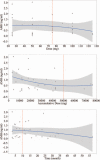
The effectiveness of thalidomide in treating inflammatory bowel disease (IBD) has been widely recognized. Meanwhile, many serious adverse drug reactions have been observed, but no know reports on ovarian reserve function.Female patients, ranging in age between 18 and 40, were referred to our institution to undergo sex hormone detection and ultrasonic scanning for ovarian function assessment, between February 1, 2016 and September 31, 2016.Thirty-three patients treated with thalidomide (group A), 73 patients without thalidomide (group B), and 78 healthy women as control were studied. Menstrual disorder was higher in group A than group B (78.8% vs 57.2%, P < 0.05), and both groups were higher than control group 33.3%, P < 0.05. Anti-Mullerian hormone (AMH) levels and antral follicle count (AFC) in group A were lower than group B, P < 0.05, while estradiol (E2) and follicle-stimulating hormone (FSH) levels were no different between 2 groups. Crohn Disease Endoscopic Index of Severity (CDEIS) and thalidomide were the independent risk factors in diminished ovarian reserve (DOR), and when dose reached 75 mg/day, 5 g total, or when treatment time reached 10 months respectively. These influence may increasing (P < 0.05), but they may recover after stopping (P < 0.05).Thalidomide was an independent risk factor leading to DOR in female IBD patients, the influence may increasing when daily dose and accumulated dose reached 75 mg/day and 5 g total dose, but may be reversed by stopping.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures
Similar articles
-
Ovarian reserve after treatment with alkylating agents during childhood.Hum Reprod. 2015 Jun;30(6):1437-46. doi: 10.1093/humrep/dev060. Epub 2015 Mar 23. Hum Reprod. 2015. PMID: 25801499
-
Ovarian reserve parameters: a comparison between users and non-users of hormonal contraception.Reprod Biomed Online. 2012 Dec;25(6):612-9. doi: 10.1016/j.rbmo.2012.09.001. Epub 2012 Sep 16. Reprod Biomed Online. 2012. PMID: 23069740
-
Ovarian reserve assessment in users of oral contraception seeking fertility advice on their reproductive lifespan.Hum Reprod. 2015 Oct;30(10):2364-75. doi: 10.1093/humrep/dev197. Epub 2015 Aug 25. Hum Reprod. 2015. PMID: 26311148
-
Testing ovarian reserve in pre-menopausal women: why, whom and how?Maturitas. 2018 Mar;109:112-117. doi: 10.1016/j.maturitas.2017.11.014. Epub 2017 Nov 22. Maturitas. 2018. PMID: 29292013 Review.
-
Ovarian response markers lead to appropriate and effective use of corifollitropin alpha in assisted reproduction.Reprod Biomed Online. 2014 Feb;28(2):183-90. doi: 10.1016/j.rbmo.2013.10.012. Epub 2013 Oct 25. Reprod Biomed Online. 2014. PMID: 24368127 Review.
Cited by
-
Ovarian reserve and IVF outcomes in patients with inflammatory bowel disease: A systematic review and meta-analysis.EClinicalMedicine. 2022 Jul 1;50:101517. doi: 10.1016/j.eclinm.2022.101517. eCollection 2022 Aug. EClinicalMedicine. 2022. PMID: 35812999 Free PMC article.
-
The efficacy and safety of thalidomide in the treatment of refractory Crohn's disease in adults: a double-center, double-blind, randomized-controlled trial.Gastroenterol Rep (Oxf). 2022 Oct 20;10:goac052. doi: 10.1093/gastro/goac052. eCollection 2022. Gastroenterol Rep (Oxf). 2022. PMID: 36284737 Free PMC article.
-
Inhibin B and antiMüllerian hormone as surrogate markers of fertility in male and female Crohn's disease patients: a case-control study.Front Med (Lausanne). 2024 Apr 25;11:1374603. doi: 10.3389/fmed.2024.1374603. eCollection 2024. Front Med (Lausanne). 2024. PMID: 38725465 Free PMC article.
-
Efficacy and safety of low-dose thalidomide combined with mesalazine in the treatment of refractory ulcerative colitis in adults.Gastroenterol Rep (Oxf). 2022 Aug 12;10:goac032. doi: 10.1093/gastro/goac032. eCollection 2022. Gastroenterol Rep (Oxf). 2022. PMID: 35975242 Free PMC article. No abstract available.
-
Clinical characteristics and risk factors of ovarian reserve decreases in women with Crohn's disease: a case-control study.J Ovarian Res. 2023 Feb 7;16(1):34. doi: 10.1186/s13048-023-01112-6. J Ovarian Res. 2023. PMID: 36750949 Free PMC article.
References
-
- McBride WG. Thalidomide and congenital abnormalities. Lancet 1961;7216:1358.
-
- Sheskin J. Thalidomide in the treatment of lepra reactions. Clin Pharmacol Ther 1965;6:303–6. - PubMed
-
- Highleyman L. FDA approves fomivirsen, famciclovir, and Thalidomide. Food and Drug Administration. BETA 1998;10:5. - PubMed
-
- Franks ME, Macpherson GR, Figg WD. Thalidomide. Lancet 2004;9423:1802–11. - PubMed
-
- Plamondon S, Ng SC, Kamm MA. Thalidomide in luminal and fistulizing Crohn's disease resistant to standard therapies. Aliment Pharmacol Ther 2007;5:557–67. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources