The Peroxisome-Mitochondria Connection: How and Why?
- PMID: 28538669
- PMCID: PMC5485950
- DOI: 10.3390/ijms18061126
The Peroxisome-Mitochondria Connection: How and Why?
Abstract
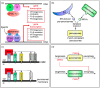
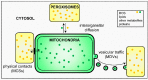
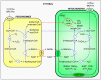
Over the past decades, peroxisomes have emerged as key regulators in overall cellular lipid and reactive oxygen species metabolism. In mammals, these organelles have also been recognized as important hubs in redox-, lipid-, inflammatory-, and innate immune-signaling networks. To exert these activities, peroxisomes must interact both functionally and physically with other cell organelles. This review provides a comprehensive look of what is currently known about the interconnectivity between peroxisomes and mitochondria within mammalian cells. We first outline how peroxisomal and mitochondrial abundance are controlled by common sets of cis- and trans-acting factors. Next, we discuss how peroxisomes and mitochondria may communicate with each other at the molecular level. In addition, we reflect on how these organelles cooperate in various metabolic and signaling pathways. Finally, we address why peroxisomes and mitochondria have to maintain a healthy relationship and why defects in one organelle may cause dysfunction in the other. Gaining a better insight into these issues is pivotal to understanding how these organelles function in their environment, both in health and disease.
Keywords: fatty acid oxidation; human disease; interorganelle crosstalk; mitochondria; organelle abundance; organelle dysfunction; peroxisomes; reactive oxygen species.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Peroxisomal metabolism and oxidative stress.Biochimie. 2014 Mar;98:56-62. doi: 10.1016/j.biochi.2013.07.026. Epub 2013 Aug 9. Biochimie. 2014. PMID: 23933092 Review.
-
Multi-localized Proteins: The Peroxisome-Mitochondria Connection.Subcell Biochem. 2018;89:383-415. doi: 10.1007/978-981-13-2233-4_17. Subcell Biochem. 2018. PMID: 30378033 Review.
-
Peroxisomal Hydrogen Peroxide Metabolism and Signaling in Health and Disease.Int J Mol Sci. 2019 Jul 26;20(15):3673. doi: 10.3390/ijms20153673. Int J Mol Sci. 2019. PMID: 31357514 Free PMC article. Review.
-
Organelle interplay in peroxisomal disorders.Trends Mol Med. 2009 Jul;15(7):293-302. doi: 10.1016/j.molmed.2009.05.002. Epub 2009 Jun 26. Trends Mol Med. 2009. PMID: 19560974 Review.
-
Role of peroxisomes in ROS/RNS-metabolism: implications for human disease.Biochim Biophys Acta. 2012 Sep;1822(9):1363-73. doi: 10.1016/j.bbadis.2011.12.001. Epub 2011 Dec 9. Biochim Biophys Acta. 2012. PMID: 22178243 Review.
Cited by
-
Functions of ROS in Macrophages and Antimicrobial Immunity.Antioxidants (Basel). 2021 Feb 19;10(2):313. doi: 10.3390/antiox10020313. Antioxidants (Basel). 2021. PMID: 33669824 Free PMC article. Review.
-
Distinct ultrastructural phenotypes of glial and neuronal alpha-synuclein inclusions in multiple system atrophy.Brain. 2024 Nov 4;147(11):3727-3741. doi: 10.1093/brain/awae137. Brain. 2024. PMID: 38696728 Free PMC article.
-
Defective mitochondria remodelling in B cells leads to an aged immune response.Nat Commun. 2024 Mar 22;15(1):2569. doi: 10.1038/s41467-024-46763-1. Nat Commun. 2024. PMID: 38519473 Free PMC article.
-
Mitochondrial sulfide promotes life span and health span through distinct mechanisms in developing versus adult treated Caenorhabditis elegans.Proc Natl Acad Sci U S A. 2023 Aug 8;120(32):e2216141120. doi: 10.1073/pnas.2216141120. Epub 2023 Jul 31. Proc Natl Acad Sci U S A. 2023. PMID: 37523525 Free PMC article.
-
Metabolomics Analyses in High-Low Feed Efficient Dairy Cows Reveal Novel Biochemical Mechanisms and Predictive Biomarkers.Metabolites. 2019 Jul 23;9(7):151. doi: 10.3390/metabo9070151. Metabolites. 2019. PMID: 31340509 Free PMC article.
References
-
- Fransen M. Peroxisome dynamics: Molecular players, mechanisms, and (dys) functions. ISRN Cell Biol. 2012;2012:714192. doi: 10.5402/2012/714192. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

