Cryo-EM structure of the activated GLP-1 receptor in complex with a G protein
- PMID: 28538729
- PMCID: PMC5587415
- DOI: 10.1038/nature22394
Cryo-EM structure of the activated GLP-1 receptor in complex with a G protein
Abstract
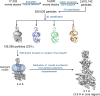
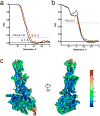
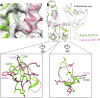
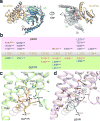
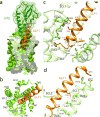
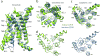
Glucagon-like peptide 1 (GLP-1) is a hormone with essential roles in regulating insulin secretion, carbohydrate metabolism and appetite. GLP-1 effects are mediated through binding to the GLP-1 receptor (GLP-1R), a class B G-protein-coupled receptor (GPCR) that signals primarily through the stimulatory G protein Gs. Class B GPCRs are important therapeutic targets; however, our understanding of their mechanism of action is limited by the lack of structural information on activated and full-length receptors. Here we report the cryo-electron microscopy structure of the peptide-activated GLP-1R-Gs complex at near atomic resolution. The peptide is clasped between the N-terminal domain and the transmembrane core of the receptor, and further stabilized by extracellular loops. Conformational changes in the transmembrane domain result in a sharp kink in the middle of transmembrane helix 6, which pivots its intracellular half outward to accommodate the α5-helix of the Ras-like domain of Gs. These results provide a structural framework for understanding class B GPCR activation through hormone binding.
Figures



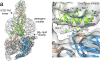


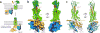


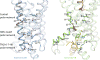
Comment in
-
A sugar high in structural biology.Nature. 2017 Jun 7;546(7657):186. doi: 10.1038/546186a. Nature. 2017. PMID: 28593982 No abstract available.
-
Once Blind, Now We See GLP-1 Molecular Action.Cell Metab. 2017 Aug 1;26(2):289-291. doi: 10.1016/j.cmet.2017.07.014. Cell Metab. 2017. PMID: 28768169
References
-
- Drucker DJ. The Cardiovascular Biology of Glucagon-like Peptide-1. Cell Metab. 2016;24:15–30. - PubMed
-
- Cho YM, Merchant CE, Kieffer TJ. Targeting the glucagon receptor family for diabetes and obesity therapy. Pharmacol. Ther. 2012;135:247–278. - PubMed
-
- Lagerstrom MC, Schioth HB. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat. Rev. Drug Discov. 2008;7:339–357. - PubMed
-
- Runge S, Thogersen H, Madsen K, Lau J, Rudolph R. Crystal structure of the ligand-bound glucagon-like peptide-1 receptor extracellular domain. J. Biol. Chem. 2008;283:11340–11347. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

