Do anti-malarials in Africa meet quality standards? The market penetration of non quality-assured artemisinin combination therapy in eight African countries
- PMID: 28539125
- PMCID: PMC5444102
- DOI: 10.1186/s12936-017-1818-8
Do anti-malarials in Africa meet quality standards? The market penetration of non quality-assured artemisinin combination therapy in eight African countries
Abstract
Background: Quality of artemisinin-based combination therapy (ACT) is important for ensuring malaria parasite clearance and protecting the efficacy of artemisinin-based therapies. The extent to which non quality-assured ACT (non-QAACT), or those not granted global regulatory approval, are available and used to treat malaria in endemic countries is poorly documented. This paper uses national and sub-national medicine outlet surveys conducted in eight study countries (Benin, Kinshasa and Kantanga [Democratic Republic of the Congo, DRC], Kenya, Madagascar, Nigeria, Tanzania, Uganda and Zambia) between 2009 and 2015 to describe the non-QAACT market and to document trends in availability and distribution of non-QAACT in the public and private sector.
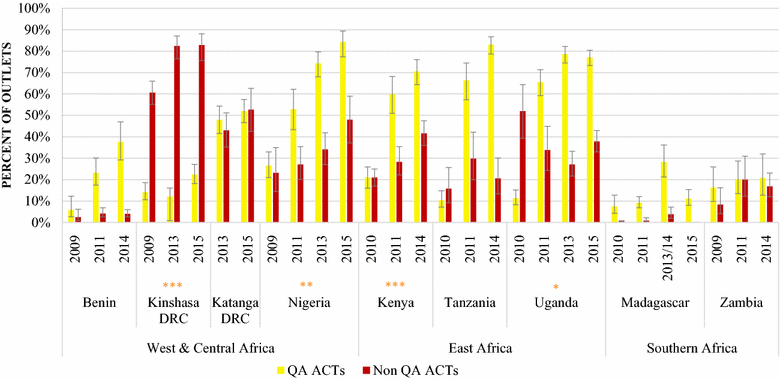
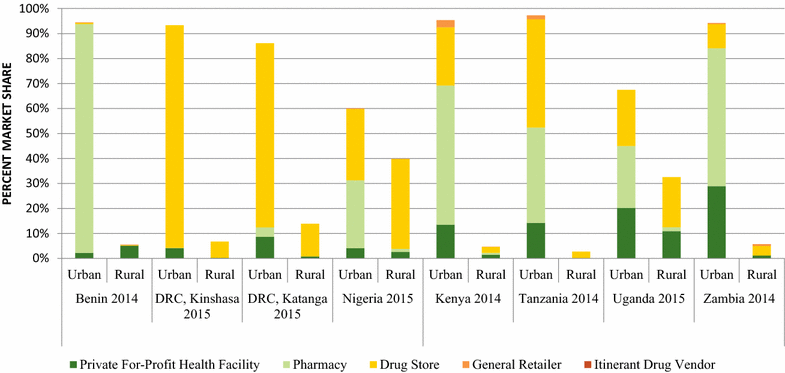
Results: In 2014/15, non-QAACT were most commonly available in Kinshasa (83%), followed by Katanga (53%), Nigeria (48%), Kenya (42%), and Uganda (33%). Non-QAACT accounted for 20% of the market share in the private sector in Kenya, followed by Benin and Uganda (19%), Nigeria (12%) and Zambia (8%); this figure was 27% in Katanga and 40% in Kinshasa. Public sector non-QAACT availability and distribution was much lower, with the exception of Zambia (availability, 85%; market share, 32%). Diverse generics and formulations were available, but non-QAACT were most commonly artemether-lumefantrine (AL) or dihydroartemisinin-piperaquine (DHA PPQ), in tablet formulation, imported, and distributed in urban areas at either pharmacies or drug stores. The number of unique manufacturers supplying non-QAACT to each country ranged from 9 in Uganda to 92 in Nigeria.
Conclusions: Addressing the availability and distribution of non-QAACT will require effective private sector engagement and evidence-based strategies to address provider and consumer demand for these products. Given the variation in non-QAACT markets observed across the eight study countries, active efforts to limit registration, importation and distribution of non-QAACT must be tailored to the country context, and will involve addressing complex and challenging aspects of medicine registration, private sector pharmaceutical regulation, local manufacturing and drug importation. These efforts may be critical not only to patient health and safety, but also to effective malaria control and protection of artemisinin drug efficacy in the face of spreading resistance.
Keywords: ACT; Anti-malarial; Medicine quality; Regulation.
Figures






References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources

