Sleep Loss Promotes Astrocytic Phagocytosis and Microglial Activation in Mouse Cerebral Cortex
- PMID: 28539349
- PMCID: PMC5456108
- DOI: 10.1523/JNEUROSCI.3981-16.2017
Sleep Loss Promotes Astrocytic Phagocytosis and Microglial Activation in Mouse Cerebral Cortex
Abstract
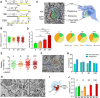
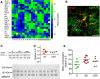
We previously found that Mertk and its ligand Gas6, astrocytic genes involved in phagocytosis, are upregulated after acute sleep deprivation. These results suggested that astrocytes may engage in phagocytic activity during extended wake, but direct evidence was lacking. Studies in humans and rodents also found that sleep loss increases peripheral markers of inflammation, but whether these changes are associated with neuroinflammation and/or activation of microglia, the brain's resident innate immune cells, was unknown. Here we used serial block-face scanning electron microscopy to obtain 3D volume measurements of synapses and surrounding astrocytic processes in mouse frontal cortex after 6-8 h of sleep, spontaneous wake, or sleep deprivation (SD) and after chronic (∼5 d) sleep restriction (CSR). Astrocytic phagocytosis, mainly of presynaptic components of large synapses, increased after both acute and chronic sleep loss relative to sleep and wake. MERTK expression and lipid peroxidation in synaptoneurosomes also increased to a similar extent after short and long sleep loss, suggesting that astrocytic phagocytosis may represent the brain's response to the increase in synaptic activity associated with prolonged wake, clearing worn components of heavily used synapses. Using confocal microscopy, we then found that CSR but not SD mice show morphological signs of microglial activation and enhanced microglial phagocytosis of synaptic elements, without obvious signs of neuroinflammation in the CSF. Because low-level sustained microglia activation can lead to abnormal responses to a secondary insult, these results suggest that chronic sleep loss, through microglia priming, may predispose the brain to further damage.SIGNIFICANCE STATEMENT We find that astrocytic phagocytosis of synaptic elements, mostly of presynaptic origin and in large synapses, is upregulated already after a few hours of sleep deprivation and shows a further significant increase after prolonged and severe sleep loss, suggesting that it may promote the housekeeping of heavily used and strong synapses in response to the increased neuronal activity of extended wake. By contrast, chronic sleep restriction but not acute sleep loss activates microglia, promotes their phagocytic activity, and does so in the absence of overt signs of neuroinflammation, suggesting that like many other stressors, extended sleep disruption may lead to a state of sustained microglia activation, perhaps increasing the brain's susceptibility to other forms of damage.
Keywords: astrocyte; cortex; microglia; mouse; sleep; sleep deprivation.
Copyright © 2017 the authors 0270-6474/17/375263-11$15.00/0.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
