Synthesis of the Ca2+-mobilizing messengers NAADP and cADPR by intracellular CD38 enzyme in the mouse heart: Role in β-adrenoceptor signaling
- PMID: 28539361
- PMCID: PMC5555186
- DOI: 10.1074/jbc.M117.789347
Synthesis of the Ca2+-mobilizing messengers NAADP and cADPR by intracellular CD38 enzyme in the mouse heart: Role in β-adrenoceptor signaling
Abstract
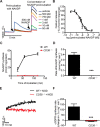
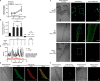
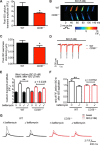
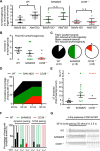
Nicotinic acid adenine dinucleotide phosphate (NAADP) and cyclic ADP-ribose (cADPR) are Ca2+-mobilizing messengers important for modulating cardiac excitation-contraction coupling and pathophysiology. CD38, which belongs to the ADP-ribosyl cyclase family, catalyzes synthesis of both NAADP and cADPR in vitro However, it remains unclear whether this is the main enzyme for their production under physiological conditions. Here we show that membrane fractions from WT but not CD38-/- mouse hearts supported NAADP and cADPR synthesis. Membrane permeabilization of cardiac myocytes with saponin and/or Triton X-100 increased NAADP synthesis, indicating that intracellular CD38 contributes to NAADP production. The permeabilization also permitted immunostaining of CD38, with a striated pattern in WT myocytes, whereas CD38-/- myocytes and nonpermeabilized WT myocytes showed little or no staining, without striation. A component of β-adrenoreceptor signaling in the heart involves NAADP and lysosomes. Accordingly, in the presence of isoproterenol, Ca2+ transients and contraction amplitudes were smaller in CD38-/- myocytes than in the WT. In addition, suppressing lysosomal function with bafilomycin A1 reduced the isoproterenol-induced increase in Ca2+ transients in cardiac myocytes from WT but not CD38-/- mice. Whole hearts isolated from CD38-/- mice and exposed to isoproterenol showed reduced arrhythmias. SAN4825, an ADP-ribosyl cyclase inhibitor that reduces cADPR and NAADP synthesis in mouse membrane fractions, was shown to bind to CD38 in docking simulations and reduced the isoproterenol-induced arrhythmias in WT hearts. These observations support generation of NAADP and cADPR by intracellular CD38, which contributes to effects of β-adrenoreceptor stimulation to increase both Ca2+ transients and the tendency to disturb heart rhythm.
Keywords: CD38; Ca2+; cardiac arrhythmia; cardiac hypertrophy; cyclic ADP-ribose (cADPR); heart; lysosomes; nicotinic acid adenine dinucleotide phosphate (NAADP); sarcoplasmic reticulum (SR); β-adrenoceptor.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article.
Figures

References
-
- Ferrero E., Lo Buono N., Horenstein A. L., Funaro A., and Malavasi F. (2014) The ADP-ribosyl cyclases: the current evolutionary state of the ARCs. Front. Biosci. 19, 986–1002 - PubMed
-
- Howard M., Grimaldi J. C., Bazan J. F., Lund F. E., Santos-Argumedo L., Parkhouse R. M., Walseth T. F., and Lee H. C. (1993) Formation and hydrolysis of cyclic ADP-ribose catalyzed by lymphocyte antigen CD38. Science 262, 1056–1059 - PubMed
-
- Aarhus R., Graeff R. M., Dickey D. M., Walseth T. F., and Lee H. C. (1995) ADP-ribosyl cyclase and CD38 catalyze the synthesis of a calcium-mobilizing metabolite from NADP. J. Biol. Chem. 270, 30327–30333 - PubMed
-
- Lee H. C. (2001) Physiological functions of cyclic ADP-ribose and NAADP as calcium messengers. Annu. Rev. Pharmacol. Toxicol. 41, 317–345 - PubMed
-
- Galione A. (2015) A primer of NAADP-mediated Ca2+ signalling: from sea urchin eggs to mammalian cells. Cell Calcium 58, 27–47 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

