The brain microenvironment mediates resistance in luminal breast cancer to PI3K inhibition through HER3 activation
- PMID: 28539475
- PMCID: PMC5917603
- DOI: 10.1126/scitranslmed.aal4682
The brain microenvironment mediates resistance in luminal breast cancer to PI3K inhibition through HER3 activation
Erratum in
-
Erratum for the Research Article: "The brain microenvironment mediates resistance in luminal breast cancer to PI3K inhibition through HER3 activation" by D. P. Kodack, V. Askoxylakis, G. B. Ferraro, Q. Sheng, M. Badeaux, S. Goel, X. Qi, R. Shankaraiah, Z. A. Cao, R. R. Ramjiawan, D. Bezwada, B. Patel, Y. Song, C. Costa, K. Naxerova, C. S. F. Wong, J. Kloepper, R. Das, A. Tam, J. Tanboon, D. G. Duda, C. R. Miller, M. B. Siegel, C. K. Anders, M. Sanders, M. V. Estrada, R. Schlegel, C. L. Arteaga, E. Brachtel, A. Huang, D. Fukumura, J. A. Engelman, R. K. Jain.Sci Transl Med. 2019 Jan 30;11(477):eaaw6935. doi: 10.1126/scitranslmed.aaw6935. Sci Transl Med. 2019. PMID: 30700575 No abstract available.
Abstract
Although targeted therapies are often effective systemically, they fail to adequately control brain metastases. In preclinical models of breast cancer that faithfully recapitulate the disparate clinical responses in these microenvironments, we observed that brain metastases evade phosphatidylinositide 3-kinase (PI3K) inhibition despite drug accumulation in the brain lesions. In comparison to extracranial disease, we observed increased HER3 expression and phosphorylation in brain lesions. HER3 blockade overcame the resistance of HER2-amplified and/or PIK3CA-mutant breast cancer brain metastases to PI3K inhibitors, resulting in marked tumor growth delay and improvement in mouse survival. These data provide a mechanistic basis for therapeutic resistance in the brain microenvironment and identify translatable treatment strategies for HER2-amplified and/or PIK3CA-mutant breast cancer brain metastases.
Copyright © 2017, American Association for the Advancement of Science.
Conflict of interest statement
Figures
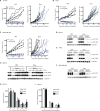

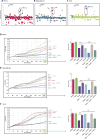
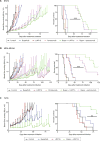
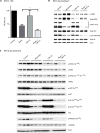
References
-
- Murrell DH, Foster PJ, Chambers AF. Brain metastases from breast cancer: Lessons from experimental magnetic resonance imaging studies and clinical implications. J Mol Med. 2014;92:5–12. - PubMed
-
- Tamura K, Kurihara H, Yonemori K, Tsuda H, Suzuki J, Kono Y, Honda N, Kodaira M, Yamamoto H, Yunokawa M, Shimizu C, Hasegawa K, Kanayama Y, Nozaki S, Kinoshita T, Wada Y, Tazawa S, Takahashi K, Watanabe Y, Fujiwara Y. 64Cu-DOTA-trastuzumab PET imaging in patients with HER2-positive breast cancer. J Nucl Med. 2013;54:1869–1875. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

