Secondary metabolite genes encoded by potato rhizosphere microbiomes in the Andean highlands are diverse and vary with sampling site and vegetation stage
- PMID: 28539610
- PMCID: PMC5443786
- DOI: 10.1038/s41598-017-02314-x
Secondary metabolite genes encoded by potato rhizosphere microbiomes in the Andean highlands are diverse and vary with sampling site and vegetation stage
Abstract
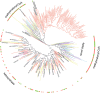
Potato (Solanum tuberosum) is an important staple crop worldwide, it has been cultivated in the Andean Altiplano under low-input farming practices at high altitudes and under harsh environment for centuries. We analyzed secondary metabolite (SM) gene diversity encoded in the potato rhizosphere microbiome during plant growth at three distinct sites located in the Andes at high altitudes by 454-pyrosequencing of non-ribosomal peptide and polyketide biosynthetic genes. Phylogenetic analysis indicated that the majority of rhizosphere SM-encoding sequences differed from previously known sequences and may have distinct ancestors. In particular, actinobacterial methyl-malonyl-CoA transferase and acyl carrier protein from Firmicutes, both involved in the synthesis of SMs, showed widespread distribution of clades which were clearly distinct from sequences deposited in public databases, and only 11% of these sequences could be linked to the production of specific classes of SMs. Although the same cultivar was analyzed, SM gene composition radically differed among plant growth stages and across sites, suggesting a distinct repertoire of SM genes that likely encode diverse SM structures. Also, great diversity of non-ribosomal peptide and polyketide biosynthetic pathways in potato-associated microbiomes in the Andean highlands may represent a rich source of novel natural products.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

