An episomal vector-based CRISPR/Cas9 system for highly efficient gene knockout in human pluripotent stem cells
- PMID: 28539611
- PMCID: PMC5443789
- DOI: 10.1038/s41598-017-02456-y
An episomal vector-based CRISPR/Cas9 system for highly efficient gene knockout in human pluripotent stem cells
Erratum in
-
Author Correction: An episomal vector-based CRISPR/Cas9 system for highly efficient gene knockout in human pluripotent stem cells.Sci Rep. 2018 Dec 12;8(1):17900. doi: 10.1038/s41598-018-36738-w. Sci Rep. 2018. PMID: 30538257 Free PMC article.
Abstract
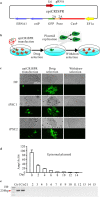
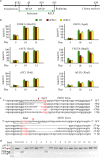
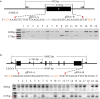
Human pluripotent stem cells (hPSCs) represent a unique opportunity for understanding the molecular mechanisms underlying complex traits and diseases. CRISPR/Cas9 is a powerful tool to introduce genetic mutations into the hPSCs for loss-of-function studies. Here, we developed an episomal vector-based CRISPR/Cas9 system, which we called epiCRISPR, for highly efficient gene knockout in hPSCs. The epiCRISPR system enables generation of up to 100% Insertion/Deletion (indel) rates. In addition, the epiCRISPR system enables efficient double-gene knockout and genomic deletion. To minimize off-target cleavage, we combined the episomal vector technology with double-nicking strategy and recent developed high fidelity Cas9. Thus the epiCRISPR system offers a highly efficient platform for genetic analysis in hPSCs.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

