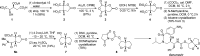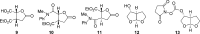Practical Synthesis of the Bicyclic Darunavir Side Chain: (3 R,3a S,6a R)-Hexahydrofuro[2,3- b]furan-3-ol from Monopotassium Isocitrate
- PMID: 28539755
- PMCID: PMC5437813
- DOI: 10.1021/acs.oprd.6b00377
Practical Synthesis of the Bicyclic Darunavir Side Chain: (3 R,3a S,6a R)-Hexahydrofuro[2,3- b]furan-3-ol from Monopotassium Isocitrate
Abstract
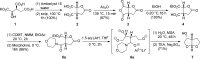
A practical synthesis of (3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-ol-a key intermediate in the synthesis of darunavir-from monopotassium isocitrate is described. The isocitric acid salt, obtained from a high-yielding fermentation fed by sunflower oil, was converted in several steps to a tertiary amide. This amide, along with the compound's ester functionalities, was reduced with lithium aluminum hydride to give, on acidic workup, a transient aminal-triol. This was converted in situ to the title compound, the bicyclic acetal furofuranol side chain of darunavir, a protease inhibitor used in treatment of HIV/AIDS. Key to the success of this process was identifying an optimal amide that allowed for complete reaction and successful product isolation. N-Methyl aniline amide was identified as the most suitable substrate for the reduction and the subsequent cyclization to the desired product. Thus, the side chain is produced in 55% overall yield from monopotassium isocitrate.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- NIH Guidelines: http://aidsinfo.nih.gov/guidelines (retrieved 22 Sep 2016); WHO Guidelines: http://www.who.int/hiv/pub/arv/arv-2016/en/ (published June 2016).
-
- Ghosh A. K.; Chen Y. Tetrahedron Lett. 1995, 36, 505.10.1016/0040-4039(94)02296-N. - DOI - PMC - PubMed
- Martin M. T.; Roberts J. C.; Toczko J. F., US 2006/0148865 A1.
- Khmelnitsky Y. L.; Michels P. C.; Cotterill I. C.; Eissenstat M.; Sunku V.; Veeramaneni V. R.; Cittineni H.; Kotha G. R.; Talasani S. R.; Ramanathan K. K.; Chitineni V. K.; Venepalli B. R. Org. Process Res. Dev. 2011, 15, 279.10.1021/op100254z. - DOI
-
- Black D. M.; Davis R.; Doan B. D.; Lovelace T. C.; Millar A.; Toczko J. F.; Xie S. Tetrahedron: Asymmetry 2008, 19, 2015.10.1016/j.tetasy.2008.07.037. - DOI
-
- Hayashi Y.; Aikawa T.; Shimasaki Y.; Okamoto H.; Tomioka Y.; Miki T.; Takeda M.; Ikemoto T. Org. Process Res. Dev. 2016, 20, 1615.10.1021/acs.oprd.6b00178. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources