Differential effects of amyloid-β peptide aggregation status on in vivo retinal neurotoxicity
- PMID: 28539771
- PMCID: PMC5436173
- DOI: 10.2147/eb.s9902
Differential effects of amyloid-β peptide aggregation status on in vivo retinal neurotoxicity
Abstract
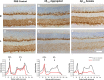
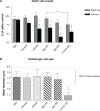
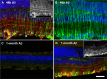
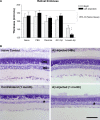
The present study examined the relationship between amyloid beta (Aβ)-peptide aggregation state and neurotoxicity in vivo using the rat retinal-vitreal model. Following single unilateral intravitreal injection of either soluble Aβ1-42 or Aβ1-42 preaggregated for different periods, retinal pathology was evaluated at 24 hours, 48 hours, and 1-month postinjection. Injection of either soluble Aβ (sAβ) or preaggregated Aβ induced a rapid reduction in immunoreactivity (IR) for synaptophysin, suggesting that direct contact with neurons is not necessary to disrupt synapses. Acute neuronal ionic and metabolic dysfunction was demonstrated by widespread loss of IR to the calcium buffering protein parvalbumin (PV) and protein gene product 9.5, a component of the ubiquitin-proteosome system. Injection of sAβ appeared to have a more rapid impact on PV than the preaggregated treatments, producing a marked reduction in PV cell diameters at 48 hours, an effect that was only observed for preaggregated Aβ after 1-month survival. Extending the preaggregation period from 4 to 8 days to obtain highly fibrillar Aβ species significantly increased the loss of choline acteyltransferase IR, but had no effect on PV-IR. These findings prompt the conclusion that Aβ assembly state has a significant impact on in vivo neurotoxicity by triggering distinct molecular changes within the cell.
Keywords: Alzheimer disease; retina.
Conflict of interest statement
Disclosure The authors declare that there are no conflicts of interest in this work.
Figures





References
-
- Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. - PubMed
-
- Burdick D, Soreghan B, Kwon M, et al. Assembly and aggregation properties of synthetic Alzheimer’s A4/beta amyloid peptide analogs. J Biol Chem. 1992;267:546–554. - PubMed
-
- Hardy J, Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol Sci. 1991;12:383–388. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

