Low-level light therapy of the eye and brain
- PMID: 28539775
- PMCID: PMC5436183
- DOI: 10.2147/EB.S21391
Low-level light therapy of the eye and brain
Abstract
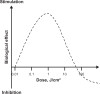
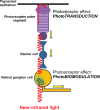
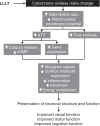
Low-level light therapy (LLLT) using red to near-infrared light energy has gained attention in recent years as a new scientific approach with therapeutic applications in ophthalmology, neurology, and psychiatry. The ongoing therapeutic revolution spearheaded by LLLT is largely propelled by progress in the basic science fields of photobiology and bioenergetics. This paper describes the mechanisms of action of LLLT at the molecular, cellular, and nervous tissue levels. Photoneuromodulation of cytochrome oxidase activity is the most important primary mechanism of action of LLLT. Cytochrome oxidase is the primary photoacceptor of light in the red to near-infrared region of the electromagnetic spectrum. It is also a key mitochondrial enzyme for cellular bioenergetics, especially for nerve cells in the retina and the brain. Evidence shows that LLLT can secondarily enhance neural metabolism by regulating mitochondrial function, intraneuronal signaling systems, and redox states. Current knowledge about LLLT dosimetry relevant for its hormetic effects on nervous tissue, including noninvasive in vivo retinal and transcranial effects, is also presented. Recent research is reviewed that supports LLLT potential benefits in retinal disease, stroke, neurotrauma, neurodegeneration, and memory and mood disorders. Since mitochondrial dysfunction plays a key role in neurodegeneration, LLLT has potential significant applications against retinal and brain damage by counteracting the consequences of mitochondrial failure. Upon transcranial delivery in vivo, LLLT induces brain metabolic and antioxidant beneficial effects, as measured by increases in cytochrome oxidase and superoxide dismutase activities. Increases in cerebral blood flow and cognitive functions induced by LLLT have also been observed in humans. Importantly, LLLT given at energy densities that exert beneficial effects does not induce adverse effects. This highlights the value of LLLT as a novel paradigm to treat visual, neurological, and psychological conditions, and supports that neuronal energy metabolism could constitute a major target for neurotherapeutics of the eye and brain.
Keywords: cognitive and mood disorders; cytochrome oxidase; neurological disease; neurotherapeutics; photobiomodulation; retinal disease.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures
References
-
- Sommer AP, Pinheiro AL, Mester AR, Franke RP, Whelan HT. Biostimulatory windows in low-intensity laser activation: lasers, scanners, and NASA’s light-emitting diode array system. J Clin Laser Med Surg. 2001;19(1):29–33. - PubMed
-
- Karu TI. Mitochondrial signaling in mammalian cells activated by red and near-IR radiation. Photochem Photobiol. 2008;84(5):1091–1099. - PubMed
-
- Morton CA, McKenna KE, Rhodes LE. Guidelines for topical photodynamic therapy: update. Br J Dermatol. 2008;159(6):1245–1266. - PubMed
-
- Fork RL. Laser stimulation of nerve cells in Aplysia. Science. 1971;171(974):907–908. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

