Targeting VEGF in canine oxygen-induced retinopathy - a model for human retinopathy of prematurity
- PMID: 28539802
- PMCID: PMC5398743
- DOI: 10.2147/EB.S94443
Targeting VEGF in canine oxygen-induced retinopathy - a model for human retinopathy of prematurity
Abstract
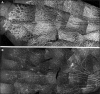
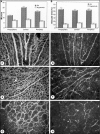
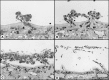
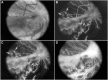
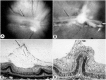
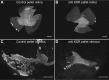
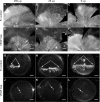
Development of the dog superficial retinal vasculature is similar to the mechanism of human retinal vasculature development; they both develop by vasculogenesis, differentiation, and assembly of vascular precursors called angioblasts. Canine oxygen-induced retinopathy (OIR) was first developed by Arnall Patz in an effort to experimentally determine the effects of hyperoxia on the development of the retinal vasculature. The canine OIR model has many characteristics in common with human retinopathy of prematurity. Exposure of 1-day-old dogs to hyperoxia for 4 days causes a vaso-obliteration throughout the retina. Vasoproliferation, after the animals have returned to room air, is robust. The initial small preretinal neovascular formations anastomose to form large preretinal membranes that eventually cause tractional retinal folds. The end-stage pathology of the canine model is similar to stage IV human retinopathy of prematurity. Therefore, canine OIR is an excellent forum to evaluate the response to drugs targeting VEGF and its receptors. Evaluation of an antibody to VEGF-R2 and the VEGF-Trap demonstrated that doses should be titered down so that preretinal neovascularization is inhibited but retinal revascularization is able to proceed, vascularizing peripheral retina and preventing it from being a source of VEGF.
Keywords: angioblasts; blood vessels; endothelial cells; oxygen; retina; retinopathy; vascular endothelial cell growth factor.
Conflict of interest statement
Disclosure The authors report no other conflict of interest in this work.
Figures






References
-
- Patz A, Eastham A, Higgenbotham D, Kleh T. Oxygen studies in retrolental fibroplasia: II. the production of the microscopic changes of retrolental fibroplasia in experimental animals. Am J Ophthalmol. 1953;36:1511–1522. - PubMed
-
- Kimura T, Chen CH, Patz A. Light and electron microscopic studies of intravitreal proliferative tissues in human and puppy eyes. Nippon Ganka Gakkai Zasshi. 1979;83:255–265. - PubMed
-
- Flower RW, Blake DA, Wajer SD, Egner PG, McLeod DS, Pitts SM. Retrolental fibroplasia: evidence for a role of the prostaglandin cascade in the pathogenesis of oxygen-induced retinopathy in the newborn beagle. Ped Res. 1981;15:1293–1302. - PubMed
- Hasegawa T, McLeod DS, Prow T, Merges C, Grebe R, Lutty GA. Vascular precursors in developing human retina. Invest Ophthalmol Vis Sci. 2008;49:2178–2192. 1. - PMC - PubMed
-
- McLeod DS, Lutty GA, Wajer SD, Flower RW. Visualization of a developing vasculature. Microvasc Res. 1987;33:257–269. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

