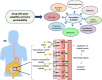
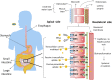
Understanding peroral absorption: regulatory aspects and contemporary approaches to tackling solubility and permeability hurdles
- PMID: 28540164
- PMCID: PMC5430883
- DOI: 10.1016/j.apsb.2016.09.005
Understanding peroral absorption: regulatory aspects and contemporary approaches to tackling solubility and permeability hurdles
Abstract
Oral drug absorption is a process influenced by the physicochemical and biopharmaceutical properties of the drug and its inter-relationship with the gastrointestinal tract. Drug solubility, dissolution and permeability across intestinal barrier are the key parameters controlling absorption. This review provides an overview of the factors that affect drug absorption and the classification of a drug on the basis of solubility and permeability. The biopharmaceutical classification system (BCS) was introduced in early 90׳s and is a regulatory tool used to predict bioavailability problems associated with a new entity, thereby helping in the development of a drug product. Strategies to combat solubility and permeability issues are also discussed.
Keywords: ABC, ATP-binding cassette; AP, absorption potential; API, active pharmaceutical ingredient; ATP, adenosine triphosphate; AZT, azidothymidine; BA/BE, bioavailability/bioequivalence; BCRP, breast cancer resistance protein; BCS; BCS, biopharmaceutical classification system; BDDS, biopharmaceutical drug disposition system; BSP, bromosulfophthalein; CD, cyclodextrin; CDER, Centre for Drug Evaluation and Research; CNT, Na+-dependent concentrative transporter; CNT, concentrative nucleoside transporter; CYP, cytochrome P450; D:S, dose:solubility; E217G, estradiol 17β-glucuronide; EMEA, European Medicines Agency; ENT, equilibrative nucleoside transporter; FATP, fatty acid transporter protein; FDA, U.S. Food and Drug Administration; FIP, International Pharmaceutical Federation; FaSSIF, fasted state simulated intestinal fluid; Factors affecting absorption; FeSSIF, fed state simulated intestinal fluid; Formulation strategies; GIS, gastrointestinal simulator; GIT, gastrointestinal tract; GITA, gastrointestinal transit and absorption; GLUT, sodium-independent facilitated diffusion transporter; GRAS, generally recognized as safe; HIV, human immunodeficiency disease; HPC-SL, LBDDS, lipid based drug delivery system; HUGO, Human Genome Organization; ICH, International Council of Harmonization; IDR, intrinsic dissolution rate; IR, immediate release; ISBT, sodium dependent bile salt transporter; MCT, monocarboxylate transporter; MPP, 1-methyl-4-phenylpyridinium; MRP, multidrug resistance associated protein; NLC, nanostructured lipid carrier; NME, new molecular entity; NTCP, sodium-dependent taurocholate co-transporting polypeptide; OAT, organic anion transporter; OATP, organic anion transporting polypeptide; OCT, organic cationic transporter; OCTN, organic cationic/carnitine transporter; OMM, ordered mesoporous material; P-gp, P-glycoprotein; PAH, p-aminohippurate; PAMPA, parallel artificial membrane permeability assay; PEG, polyethylene glycol; PEI, polyethyleneimine; PEPT, peptide transporter; PGA, polyglycolic acid; PLA, poly(lactic acid); PLGA, poly-d,l-lactide-co-glycoside; PMAT, plasma membrane monoamine transport; PSA, polar surface area; PVDF, polyvinylidene difluoride; Papp, apparent permeability; Peff, effective permeability; Permeability; Psi, porous silicon; RFC, reduced folate transporter; SDS, sodium dodecyl sulphate; SGLT, sodium dependent secondary active transporter; SIF, simulated intestinal fluid; SLC, solute carrier; SLCO, solute carrier organic anion; SLN, solid lipid nanoparticles; SMVT, sodium dependent multivitamin transporter; SPIP, single pass intestinal perfusion; SUPAC, scale-up and post approval changes; SVCT, sodium-dependent vitamin C transporter; Solubility; TEOS, tetraethylortho silicate; UWL, unstirred water layer; VDAD, volume to dissolve applied dose; WHO, World Health Organization; pMMA, polymethyl methacrylate; vit. E TPGS, vitamin E tocopherol polyethylene glycol succinate.
Figures
References
-
- Bergström C.A., Holm R., Jørgensen S.A., Andersson S.B., Artursson P., Beato S. Early pharmaceutical profiling to predict oral drug absorption: current status and unmet needs. Eur J Pharm Sci. 2014;57:173–199. - PubMed
-
- Dokoumetzidis A., Macheras P. A century of dissolution research: from noyes and whitney to the biopharmaceutics classification system. Int J Pharm. 2006;321:1–11. - PubMed
-
- Noyes A.A., Whitney W.R. The rate of solution of solid substances in their own solutions. J Am Chem Soc. 1897;19:930–934.
-
- Brünner E. Reaktionsgeschwindigkeit in heterogen systemen. Z Phys Chem. 1904;47:56–102.
-
- Hixson A.W., Crowell J.H. Dependence of reaction velocity upon surface and agitation. Ind Eng Chem. 1931;23:923–931.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous