Nanotechnology-based strategies for treatment of ocular disease
- PMID: 28540165
- PMCID: PMC5430571
- DOI: 10.1016/j.apsb.2016.09.001
Nanotechnology-based strategies for treatment of ocular disease
Abstract
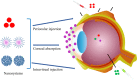
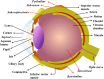
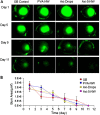
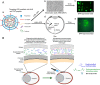
Ocular diseases include various anterior and posterior segment diseases. Due to the unique anatomy and physiology of the eye, efficient ocular drug delivery is a great challenge to researchers and pharmacologists. Although there are conventional noninvasive and invasive treatments, such as eye drops, injections and implants, the current treatments either suffer from low bioavailability or severe adverse ocular effects. Alternatively, the emerging nanoscience and nanotechnology are playing an important role in the development of novel strategies for ocular disease therapy. Various active molecules have been designed to associate with nanocarriers to overcome ocular barriers and intimately interact with specific ocular tissues. In this review, we highlight the recent attempts of nanotechnology-based systems for imaging and treating ocular diseases, such as corneal d iseases, glaucoma, retina diseases, and choroid diseases. Although additional work remains, the progress described herein may pave the way to new, highly effective and important ocular nanomedicines.
Keywords: Diagnosis; Eye; Nanocarrier; Nanosystems; Ocular disease; Ocular drug delivery; Therapy.
Figures


References
-
- Pascolini D., Mariotti S.P. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2011;96:614–618. - PubMed
-
- Schoenfeld E.R., Greene J.M., Wu S.Y., Leske M.C. Patterns of adherence to diabetes vision care guidelines: baseline findings from the diabetic retinopathy awareness program. Ophthalmology. 2001;108:563–571. - PubMed
-
- Raghava S., Goel G., Kompella U.B. Ophthalmic applications of nanotechnology. In: Phd J.T., Dphil C.J., editors. Ocular transporters in ophthalmic diseases and drug delivery. Humana Press; Totowa: 2008. pp. 415–435.
-
- Bell A.T. The impact of nanoscience on heterogeneous catalysis. Science. 2003;299:1688–1691. - PubMed
-
- Whitesides G.M. Nanoscience, nanotechnology, and chemistry. Small. 2005;1:172–179. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

