T cell--associated immunoregulation and antiviral effect of oxymatrine in hydrodynamic injection HBV mouse model
- PMID: 28540167
- PMCID: PMC5430867
- DOI: 10.1016/j.apsb.2017.02.005
T cell--associated immunoregulation and antiviral effect of oxymatrine in hydrodynamic injection HBV mouse model
Abstract
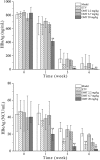
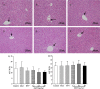
Although oxymatrine (OMT) has been shown to directly inhibit the replication of hepatitis B virus (HBV) in vitro, limited research has been done with this drug in vivo. In the present study, the antiviral effect of OMT was investigated in an immunocompetent mouse model of chronic HBV infection. The infection was achieved by tail vein injection of a large volume of DNA solution. OMT (2.2, 6.7 and 20 mg/kg) was administered by daily intraperitoneal injection for 6 weeks. The efficacy of OMT was evaluated by the levels of HBV DNA, hepatitis B surface antigen (HBsAg), hepatitis B e antigen (HBeAg) and hepatitis B core antigen (HBcAg). The immunoregulatory activity of OMT was evaluated by serum ELISA and flow cytometry. Results shows that OMT at 20 mg/kg inhibited HBV replication, and it was more efficient than entecavir (ETV) in the elimination of serum HBsAg and intrahepatic HBcAg. In addition, OMT accelerated the production of interferon-γ (IFN-γ) in a dose-dependent manner in CD4+ T cells. Our findings demonstrate the beneficial effects of OMT on the enhancement of immunological function and in the control of HBV antigens. The findings suggest this drug to be a good antiviral therapeutic candidate for the treatment of HBV infection.
Keywords: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CD4+ T cell; CHB, chronic hepatitis B; ETV, entecavir; HBV; HBV, hepatitis B virus; HBcAg, hepatitis B core antigen; HBeAg, hepatitis B e antigen; HBsAg, hepatitis B surface antigen; HE, hematoxylin and eosin; IFN-γ; IFN-γ, interferon-γ; IL-4, interleukin-4; Mouse; NAs, nucleoside and nucleotide analogs; OMT, oxymatrine; Oxymatrine; TCMs, traditional Chinese medicines; TNF-α, tumor necrosis factor-α.
Figures




Similar articles
-
Evaluation of Serum IFN- and IL-5 Levels in Response to Entecavir Therapy in Patients with Chronic Hepatitis B Virus Infection.Egypt J Immunol. 2018 Jan;25(1):93-103. Egypt J Immunol. 2018. PMID: 30243001
-
CpG oligodeoxynucleotide inhibits HBV replication in a hydrodynamic injection murine model.Antivir Ther. 2015;20(3):289-95. doi: 10.3851/IMP2870. Epub 2014 Oct 3. Antivir Ther. 2015. PMID: 25279542
-
High persistence rate of hepatitis B virus in a hydrodynamic injection-based transfection model in C3H/HeN mice.World J Gastroenterol. 2015 Mar 28;21(12):3527-36. doi: 10.3748/wjg.v21.i12.3527. World J Gastroenterol. 2015. PMID: 25834317 Free PMC article.
-
Treatment of chronic hepatitis B: case selection and duration of therapy.J Gastroenterol Hepatol. 2002 Apr;17(4):409-14. doi: 10.1046/j.1440-1746.2002.02767.x. J Gastroenterol Hepatol. 2002. PMID: 11982721 Review.
-
Progression and status of antiviral monitoring in patients with chronic hepatitis B: From HBsAg to HBV RNA.World J Hepatol. 2018 Sep 27;10(9):603-611. doi: 10.4254/wjh.v10.i9.603. World J Hepatol. 2018. PMID: 30310538 Free PMC article. Review.
Cited by
-
Mechanism of oxymatrine in the treatment of cryptosporidiosis through TNF/NF-κB signaling pathway based on network pharmacology and experimental validation.Sci Rep. 2024 Jun 24;14(1):14469. doi: 10.1038/s41598-024-65362-0. Sci Rep. 2024. PMID: 38914662 Free PMC article.
-
Metabolites from traditional Chinese botanical drugs with anti-hepatitis B virus activity - a review.Front Pharmacol. 2024 Jul 12;15:1331967. doi: 10.3389/fphar.2024.1331967. eCollection 2024. Front Pharmacol. 2024. PMID: 39070799 Free PMC article. Review.
-
The role of Traditional Chinese medicine in anti-HBV: background, progress, and challenges.Chin Med. 2023 Dec 2;18(1):159. doi: 10.1186/s13020-023-00861-2. Chin Med. 2023. PMID: 38042824 Free PMC article. Review.
-
Oxymatrine and Cisplatin Synergistically Enhance Anti-tumor Immunity of CD8+ T Cells in Non-small Cell Lung Cancer.Front Oncol. 2018 Dec 18;8:631. doi: 10.3389/fonc.2018.00631. eCollection 2018. Front Oncol. 2018. PMID: 30619765 Free PMC article.
-
Chinese Patent Medicine Liuweiwuling Tablet had Potent Inhibitory Effects on Both Wild-Type and Entecavir-Resistant Hepatitis B Virus (HBV) in vitro and Effectively Suppressed HBV Replication in Mouse Model.Front Pharmacol. 2021 Oct 27;12:756975. doi: 10.3389/fphar.2021.756975. eCollection 2021. Front Pharmacol. 2021. PMID: 34776974 Free PMC article.
References
-
- Liaw Y.F., Chu C.M. Hepatitis B virus infection. Lancet. 2009;373:582–592. - PubMed
-
- Dienstag J.L. Benefits and risks of nucleoside analog therapy for hepatitis B. Hepatology. 2009;49:S112–S121. - PubMed
-
- Chevaliez S., Hézode C., Bahrami S., Grare M., Pawlotsky J.M. Long-term hepatitis B surface antigen (HBsAg) kinetics during nucleoside/nucleotide analogue therapy: finite treatment duration unlikely. J Hepatol. 2013;58:676–683. - PubMed
-
- Cornberg M., Höner Zu Siederdissen C. HBsAg seroclearance with NUCs: rare but important. Gut. 2014;63:1208–1209. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

