The effect of 8-OH-DPAT and dapoxetine on gene expression in the brain of male rats during ejaculation
- PMID: 28540176
- PMCID: PMC5430880
- DOI: 10.1016/j.apsb.2016.11.004
The effect of 8-OH-DPAT and dapoxetine on gene expression in the brain of male rats during ejaculation
Abstract
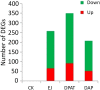
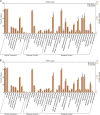
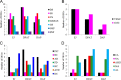
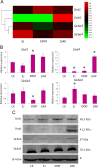
The 5-HT1A receptor agonist 8-hydroxy-2-[di-n-propylamino] tetralin (8-OH-DPAT) promotes ejaculation of male rats, whereas dapoxetine delays this process. However, the gene expression profile of the brain at ejaculation following administrationof these two compounds has not been fully elucidated. In the present study, a transcriptomic BodyMap was generated by conducting mRNA-Seq on brain samples of male Sprague-Dawley rats. The study included four groups: pre-copulatory control (CK) group, ejaculation (EJ) group, 0.5 mg/kg 8-OH-DPAT-ejaculation group (DPAT), and 60 mg/kg dapoxetine-ejaculation (DAP) group. The resulting analysis generated an average of approximately 47 million sequence reads. Significant differences in the gene expression profiles of the aforementioned groups were observed in the EJ (257 genes), DPAT (349 genes) and the DAP (207 genes) compared with the control rats. The results indicate that the expression of Drd1 and Slc6a3 was significantly different after treatment with 8-OH-DPAT, whereas the expression of Drd4 was significantly different after treatment with dapoxetine. Other genes, such as Wnt9b, Cdkn1a and Fosb, exhibited significant differences in expression after the two treatments and are related to bladder cancer, renal cell carcinoma and sexual addiction. The present study reveals the basic pattern of gene expression that was activated at ejaculation in the presence of 8-OH-DPAT or dapoxetine, providing preliminary gene expression information during rat ejaculation.
Keywords: 8-OH-DPAT; Brain; Dapoxetine; Ejaculation; Gene expression; Male rats.
Figures
References
-
- Newman H.F., Reiss H., Northup J.D. Physical basis of emission, ejaculation, and orgasm in the male. Urology. 1982;19:341–350. - PubMed
-
- deGroat W.C., Booth A.M. Physiology of male sexual function. Ann Intern Med. 1980;92:329–331. - PubMed
-
- Bole-Feysot C., Goffin V., Edery M., Binart N., Kelly P.A. Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr Rev. 1998;19:225–268. - PubMed
-
- Krüger T.H., Hartmann U., Schedlowski M. Prolactinergic and dopaminergic mechanisms underlying sexual arousal and orgasm in humans. World J Urol. 2005;23:130–138. - PubMed
-
- Hull E.M., Dominguez J.M. Getting his act together: roles of glutamate, nitric oxide, and dopamine in the medial preoptic area. Brain Res. 2006;1126:66–75. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

