Structural analysis of recombinant human ubiquitin-conjugating enzyme UbcH5c
- PMID: 28540177
- PMCID: PMC5430876
- DOI: 10.1016/j.apsb.2016.12.008
Structural analysis of recombinant human ubiquitin-conjugating enzyme UbcH5c
Abstract
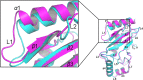
UbcH5c belongs to the ubiquitin-conjugating enzyme family and plays an important role in catalyzing ubiquitination during TNF-α--triggered NF-κB activation. Therefore, UbcH5c is a potent therapeutic target for the treatment of inflammatory and autoimmune diseases induced by aberrant activation of NF-κB. In this study, we established a stable expression system for recombinant UbcH5c and solved the crystal structure of UbcH5c belonging to space group P22121 with one molecule in the asymmetric unit. This study provides the basis for further study of UbcH5c including the design of UbcH5c inhibitors.
Keywords: Crystal structure; Inflammatory target; NF-κB; UbcH5c; Ubiquitin-conjugating enzyme; Ubiquitination.
Figures





References
-
- Chen G., Goeddel D.V. TNF-R1 signaling: a beautiful pathway. Science. 2002;296:1634–1635. - PubMed
-
- Bertrand M.J., Milutinovic S., Dickson K.M., Ho W.C., Boudreault A., Durkin J. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol Cell. 2008;30:689–700. - PubMed
-
- Ea C.K., Deng L., Xia Z.P., Pineda G., Chen Z.J. Activation of IKK by TNFα requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol Cell. 2006;22:245–257. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

