Benefits and harms of 4-factor prothrombin complex concentrate for reversal of vitamin K antagonist associated bleeding: a systematic review and meta-analysis
- PMID: 28540468
- PMCID: PMC5486892
- DOI: 10.1007/s11239-017-1506-0
Benefits and harms of 4-factor prothrombin complex concentrate for reversal of vitamin K antagonist associated bleeding: a systematic review and meta-analysis
Abstract
Prothrombin complex concentrate (PCC) is used for reversal of vitamin K antagonists (VKA) in patients with bleeding complications. This study aims to assess benefits and harms of 4-factor PCC compared to fresh frozen plasma (FFP) or no treatment in VKA associated bleeding. PubMed, EMBASE and CENTRAL were searched from 1945 to August 2015. Studies reporting 4-factor PCC use for VKA associated bleeding and providing data on INR normalization, mortality or thromboembolic (TE) complications were eligible. Two authors screened titles and full articles for inclusion, extracted data, and assessed risk of bias. Mortality data were pooled using Mantel-Haenszel random effects meta-analysis. Nineteen studies were included (N = 2878); 18 cohort studies and one RCT. Six studies had good methodological quality, 9 moderate and 4 poor. Baseline INR values ranged from 2.2 to >20. The INR within 1 h after PCC administration ranged from 1.4 to 1.9, and after FFP administration from 2.2 to 12. PCC reduced the time to reach INR correction in comparison with FFP or no treatment. The observed mortality rate ranged from 0 to 43% (mean 17%) in the PCC, 4.8-54% (mean 16%) in the FFP and 23-69% (mean 51%) in the no treatment group. Meta-analysis of mortality data resulted in an OR of 0.64 (95% confidence interval [CI] 0.27-1.5) for PCC versus FFP and an OR 0.41 (95% CI 0.13-1.3) for PCC versus no treatment. TE complications were observed in 0-18% (mean 2.5%) of PCC and in 6.4% of FFP recipients. Four-factor PCC is an effective and safe option in reversal of VKA bleeding events.
Keywords: Bleeding; Fresh frozen plasma; INR normalization; Mortality; Prothrombin complex concentrate; Vitamin K antagonist.
Conflict of interest statement
Conflict of interest
M.P.A. Brekelmans has nothing to disclose. K. van Ginkel has nothing to disclose. J.G. Daams has nothing to disclose. B.A. Hutten has nothing to disclose. S. Middeldorp reports Grant or Research Support from GSK/Aspen, BMS/Pfizer, Sanquin and Bayer, Consultant fees from Bayer, BMS/Pfizer, Boehringer Ingelheim and Daiichi Sankyo, Paid Instructor at Bayer, GSK BMS/Pfizer, Boehringer Ingelheim and Daiichi Sankyo outside the submitted work. M. Coppens has received consultancy and lecturing fees, as well as research support from Daiichi-Sankyo, Boehringer-Ingelheim, Bayer, Bristol Myers-Squibb, Pfizer and Sanquin Blood Supply outside the submitted work.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Figures
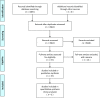
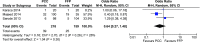
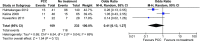
References
-
- Holbrook A, Schulman S, Witt DM, Vandvik PO, Fish J, Kovacs MJ, Svensson PJ, Veenstra DL, Crowther M, Guyatt GH. Evidence-based management of anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th edn: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:e152S–e184S. doi: 10.1378/chest.11-2295. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

