Morphometric Characterization of Rat and Human Alveolar Macrophage Cell Models and their Response to Amiodarone using High Content Image Analysis
- PMID: 28540501
- PMCID: PMC5736774
- DOI: 10.1007/s11095-017-2176-5
Morphometric Characterization of Rat and Human Alveolar Macrophage Cell Models and their Response to Amiodarone using High Content Image Analysis
Abstract
Purpose: Progress to the clinic may be delayed or prevented when vacuolated or "foamy" alveolar macrophages are observed during non-clinical inhalation toxicology assessment. The first step in developing methods to study this response in vitro is to characterize macrophage cell lines and their response to drug exposures.
Methods: Human (U937) and rat (NR8383) cell lines and primary rat alveolar macrophages obtained by bronchoalveolar lavage were characterized using high content fluorescence imaging analysis quantification of cell viability, morphometry, and phospholipid and neutral lipid accumulation.
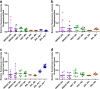
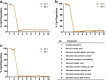
Results: Cell health, morphology and lipid content were comparable (p < 0.05) for both cell lines and the primary macrophages in terms of vacuole number, size and lipid content. Responses to amiodarone, a known inducer of phospholipidosis, required analysis of shifts in cell population profiles (the proportion of cells with elevated vacuolation or lipid content) rather than average population data which was insensitive to the changes observed.
Conclusions: A high content image analysis assay was developed and used to provide detailed morphological characterization of rat and human alveolar-like macrophages and their response to a phospholipidosis-inducing agent. This provides a basis for development of assays to predict or understand macrophage vacuolation following inhaled drug exposure.
Keywords: NR8383; U937; foamy macrophage; toxicology; vacuolation.
Figures




Similar articles
-
High Content Image Analysis of Cellular Responses of the Murine J774A.1 Cell Line and Primary Human Cells Alveolar Macrophages to an Extended Panel of Pharmaceutical Agents.Pharm Res. 2025 Jan;42(1):93-108. doi: 10.1007/s11095-024-03806-y. Epub 2025 Jan 7. Pharm Res. 2025. PMID: 39775613 Free PMC article.
-
High Content Image Analysis as a Tool to Morphologically Distinguish Macrophage Activation and Determine Its Importance for Foamy Alveolar Macrophage Responses.Front Immunol. 2021 Sep 1;12:611280. doi: 10.3389/fimmu.2021.611280. eCollection 2021. Front Immunol. 2021. PMID: 34539620 Free PMC article.
-
Profiling alveolar macrophage responses to inhaled compounds using in vitro high content image analysis.Toxicol Appl Pharmacol. 2023 Sep 1;474:116608. doi: 10.1016/j.taap.2023.116608. Epub 2023 Jun 28. Toxicol Appl Pharmacol. 2023. PMID: 37385476
-
In Vitro Multiparameter Assay Development Strategy toward Differentiating Macrophage Responses to Inhaled Medicines.Mol Pharm. 2015 Aug 3;12(8):2675-87. doi: 10.1021/acs.molpharmaceut.5b00048. Epub 2015 May 19. Mol Pharm. 2015. PMID: 25941945
-
Foamy macrophage responses in the rat lung following exposure to inhaled pharmaceuticals: a simple, pragmatic approach for inhaled drug development.J Appl Toxicol. 2014 Apr;34(4):319-31. doi: 10.1002/jat.2950. Epub 2013 Nov 6. J Appl Toxicol. 2014. PMID: 24474237 Review.
Cited by
-
Longitudinal characterization of TK6 cells sequentially adapted to animal product-free, chemically defined culture medium: considerations for genotoxicity studies.Front Toxicol. 2023 Jul 4;5:1177586. doi: 10.3389/ftox.2023.1177586. eCollection 2023. Front Toxicol. 2023. PMID: 37469456 Free PMC article.
-
Modulation of Alveolar Macrophage Activity by Eugenol Attenuates Cigarette-Smoke-Induced Acute Lung Injury in Mice.Antioxidants (Basel). 2023 Jun 11;12(6):1258. doi: 10.3390/antiox12061258. Antioxidants (Basel). 2023. PMID: 37371988 Free PMC article.
-
High Content Image Analysis of Cellular Responses of the Murine J774A.1 Cell Line and Primary Human Cells Alveolar Macrophages to an Extended Panel of Pharmaceutical Agents.Pharm Res. 2025 Jan;42(1):93-108. doi: 10.1007/s11095-024-03806-y. Epub 2025 Jan 7. Pharm Res. 2025. PMID: 39775613 Free PMC article.
-
Comparison of Oral, Intranasal and Aerosol Administration of Amiodarone in Rats as a Model of Pulmonary Phospholipidosis.Pharmaceutics. 2019 Jul 17;11(7):345. doi: 10.3390/pharmaceutics11070345. Pharmaceutics. 2019. PMID: 31319538 Free PMC article.
-
High Content Image Analysis as a Tool to Morphologically Distinguish Macrophage Activation and Determine Its Importance for Foamy Alveolar Macrophage Responses.Front Immunol. 2021 Sep 1;12:611280. doi: 10.3389/fimmu.2021.611280. eCollection 2021. Front Immunol. 2021. PMID: 34539620 Free PMC article.
References
-
- Nikula KJ, Mccartney JE, Mcgovern T, Miller GK, Odin M, Pino MV, Reed MD. STP position paper: interpreting the significance of increased alveolar macrophages in rodents following inhalation of pharmaceutical materials. Toxicologic pathology. 2014;42:472–486. doi: 10.1177/0192623313507003. - DOI - PubMed
-
- Forbes B, O'Lone R, Allen PP, Cahn A, Clarke C, Collinge M, Dailey LA, Donnelly LE, Dybowski J, Hassall D, Hildebrand D, Jones R, Kilgour J, Klapwijk J, Maier CC, Mcgovern T, Nikula K, Parry JD, Reed MD, Robinson I, Tomlinson L, Wolfreys A. Challenges for inhaled drug discovery and development: Induced alveolar macrophage responses. Advanced drug delivery reviews. 2014;71:15–33. doi: 10.1016/j.addr.2014.02.001. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

