Antithrombotic treatment after stroke due to intracerebral haemorrhage
- PMID: 28540976
- PMCID: PMC6481874
- DOI: 10.1002/14651858.CD012144.pub2
Antithrombotic treatment after stroke due to intracerebral haemorrhage
Update in
-
Antithrombotic treatment after stroke due to intracerebral haemorrhage.Cochrane Database Syst Rev. 2023 Jan 26;1(1):CD012144. doi: 10.1002/14651858.CD012144.pub3. Cochrane Database Syst Rev. 2023. PMID: 36700520 Free PMC article.
Abstract
Background: Survivors of stroke due to intracerebral haemorrhage (ICH) are at risk of thromboembolism. Antithrombotic (antiplatelet or anticoagulant) treatments may lower the risk of thromboembolism after ICH, but they may increase the risks of bleeding.
Objectives: To determine the overall effectiveness and safety of antithrombotic drugs for people with ICH.
Search methods: We searched the Cochrane Stroke Group Trials Register (24 March 2017). We also searched the Cochrane Central Register of Controlled Trials (CENTRAL: the Cochrane Library 2017, Issue 3), MEDLINE Ovid (from 1948 to March 2017), Embase Ovid (from 1980 to March 2017), and online registries of clinical trials (8 March 2017). We also screened the reference lists of included trials for additional, potentially relevant studies.
Selection criteria: We selected all randomised controlled trials (RCTs) of any antithrombotic treatment after ICH.
Data collection and analysis: Three review authors independently extracted data. We converted categorical estimates of effect to the risk ratio (RR) or odds ratio (OR), as appropriate. We divided our analyses into short- and long-term treatment, and used fixed-effect modelling for meta-analyses. Three review authors independently assessed the included RCTs for risks of bias and we created a 'Summary of findings' table using GRADE.
Main results: We included two RCTs with a total of 121 participants. Both RCTs were of short-term parenteral anticoagulation early after ICH: one tested heparin and the other enoxaparin. The risk of bias in the included RCTs was generally unclear or low, with the exception of blinding of participants and personnel, which was not done. The included RCTs did not report our chosen primary outcome (a composite outcome of all serious vascular events including ischaemic stroke, myocardial infarction, other major ischaemic event, ICH, major extracerebral haemorrhage, and vascular death). Parenteral anticoagulation did not cause a statistically significant difference in case fatality (RR 1.25, 95% confidence interval (CI) 0.38 to 4.07 in one RCT involving 46 participants, low-quality evidence), ICH, or major extracerebral haemorrhage (no detected events in one RCT involving 75 participants, low-quality evidence), growth of ICH (RR 1.64, 95% CI 0.51 to 5.29 in two RCTs involving 121 participants, low-quality evidence), deep vein thrombosis (RR 0.99, 95% CI 0.49 to 1.96 in two RCTs involving 121 participants, low quality evidence), or major ischaemic events (RR 0.54, 95% CI 0.23 to 1.28 in two RCTs involving 121 participants, low quality evidence).
Authors' conclusions: There is insufficient evidence from RCTs to support or discourage the use of antithrombotic treatment after ICH. RCTs comparing starting versus avoiding antiplatelet or anticoagulant drugs after ICH appear justified and are needed in clinical practice.
Conflict of interest statement
Luke A Perry: none known Eivind Berge: co‐ordinating investigator of the ongoing STATICH trial. Joshua Bowditch: none known Elisabeth Forfang: managing investigator of the ongoing STATICH trial. Ole Morten Rønning: none known Graeme J Hankey: in the past three years, GJH has received honoraria from AC Immune for chairing the data safety monitoring committee of two clinical trials of vaccines for Alzheimer’s disease, from Bayer for lecturing about stroke prevention in atrial fibrillation at sponsored scientific symposia, and from Medscape, Web MD for participating in a discussion about stroke prevention in atrial fibrillation for theheart.org. Elmer Villanueva: none known Rustam Al‐Shahi Salman: Chief investigator of the UK REstart or STop Antithrombotics Randomised Trial (RESTART,
Figures
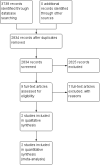








References
References to studies included in this review
Dickmann 1988 {published data only}
Orken 2009 {published data only}
References to studies excluded from this review
Boeer 1991 {published data only}
CAST 1997 {published data only}
-
- CAST (Chinese Acute Stroke Trial) Collaborative Group. CAST: randomised placebo‐controlled trial of early aspirin use in 20 000 patients with acute ischaemic stroke; CAST (Chinese Acute Stroke Trial) Collaborative Group. Lancet 1997;349(9066):1641‐9. [DOI: 10.1016/S0140-6736(97)04010-5; PUBMED: 9186381] - DOI - PubMed
Frontera 2014 {published data only}
-
- Frontera JA, Jovine M, Zach V, Gordon E. Safety of venous thromboembolism prophylaxis in intracranial hemorrhage patients with external ventricular drains. Stroke 2014;45:236.
IST 1997 {published data only}
-
- International Stroke Trial Collaborative Group. The International Stoke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19 435 patients with acute ischaemic stroke. Lancet 1997;349(9065):1569‐81. [DOI: 10.1016/S0140-6736(97)04011-7; PUBMED: 9186381] - DOI - PubMed
Li 2013 {published data only}
-
- Li X, Zhaosheng S, Zhao W, Zhang J, Chen J, Li Y, et al. Effect of acetylsalicylic acid usage and platelet transfusion on postoperative hemorrhage and activities of daily living in patients with acute intracerebral hemorrhage. Journal of Neurosurgery 2013;118:94‐103. [DOI: 10.3171/2012.9.JNS112286] - DOI - PubMed
Venturelli 2014 {published data only}
-
- Venturelli PA, Wang X, Arima H, Heeley E, Delcourt C, Lavados P, et al. Safety of prophylactic heparin in acute intracerebral haemorrhage: post‐hoc analysis of the INTERACT2 study. International Journal of Stroke 2014;9 Suppl 1:38.
Yan 2014 {published data only}
References to ongoing studies
APACHE‐AF {published data only}
-
- Nieuwenhuizen KM, Worp HB, Algra A, Kappelle LJ, Rinkel GJ, Gelder IC, et al. Apixaban versus antiplatelet drugs or no antithrombotic drugs after anticoagulation‐associated intracerebral haemorrhage in patients with atrial fibrillation (APACHE‐AF): study protocol for a randomised controlled trial. Trials 2015;16:393. [DOI: 10.1186/s13063-015-0898-4] - DOI - PMC - PubMed
NASPAF‐ICH {published data only}
-
- NCT02998905. NOACs for stroke prevention in patients with atrial fibrillation and previous ICH (NASPAF‐ICH). ClinicalTrials.gov/NCT02998905 (first received: 25 November 2016). [NCT02998905]
PICASSO {published data only}
-
- Hong KS, Kim BJ, Lee JY, Kwon SU, PICASSO Investigators. Rationale and design of the preventIon of cardiovascular events in ischemic stroke patients with high risk of cerebral hemorrhage (PICASSO) study: a randomized controlled trial. International Journal of Stroke 2015;10(7):1153‐8. [10.1111/ijs.12519; PUBMED: 26044566] - PubMed
RESTART {published data only}
-
- Al‐Shahi Salman R, Innes K, RESTART Trial Collaborators. Restart or stop antithrombotics randomised trial (RESTART). International Journal of Stroke 2015;10 Suppl 2:426. [10.1186/ISRCTN71907627]
RESTART Nord de France {published data only}
-
- Cordonnier C. RESTART Nord de France [personal communication]. Email to: LA Perry. 26 September 2016.
SoSTART {unpublished data only}
-
- Start or STop Anticoagulants Randomised Trial. Ongoing study 2017.
STATICH {published data only}
-
- Berge E. STATICH [personal communication]. Email to: LA Perry. 7 September 2016.
Additional references
Al‐Shahi Salman 2014
Béjot 2013
Chao 2016
Chong 2012
Claassen 2008
Cordonnier 2007
De Vleeschouwer 2005
-
- Vleeschouwer S, Calenbergh F, Loon J, Nuttin B, Goffin J, Plets C. Risk analysis of thrombo‐embolic and recurrent bleeding events in the management of intracranial haemorrhage due to oral anticoagulation. Acta Chirurgica Belgica 2005;105(3):268‐74. [DOI: 10.1080/00015458.2005.11679715; PUBMED: 16018519] - DOI - PubMed
Falcone 2014
-
- Falcone G, Rosand J. Aspirin should be discontinued after lobar intracerebral hemorrhage. Stroke 2014;45(10):3151‐2. [PUBMED: 10.1161/STROKEAHA.114.00578; PUBMED: 25205309] - PubMed
Flynn 2010a
Flynn 2010b
GRADEpro GDT [Computer program]
-
- McMaster University. GRADEpro Guideline Development Tool. McMaster University, 2015.
Hawryluk 2010
-
- Hawryluk GW, Austin JW, Furlan JC, Lee JB, O'Kelly C, Fehlings MG. Management of anticoagulation following central nervous system hemorrhage in patients with high thromboembolic risk. Journal of Thrombosis and Haemostasis 2010;8(7):1500. [DOI: 10.1111/j.1538-7836.2010.03882.x; PUBMED: 20403088] - DOI - PubMed
Higgins 2011
-
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Keir 2002
Kothari 1996
Krishnamurthi 2010
-
- Krishnamurthi RV, Feigin VL, Forouzanfar MH, Mensah GA, Connor M, Bennett DA, et al. GBD Stroke Experts Group. Global and regional burden of first‐ever ischaemic and haemorrhagic stroke during 1990‐2010: findings from the Global Burden of Disease Study 2010. Lancet Global Health 2013;1(5):259‐81. [DOI: 10.1016/S2214-109X(13)70089-5] - DOI - PMC - PubMed
Kuramatsu 2015
Lauer 2013
Lovelock 2007
Molina 2011
-
- Molina CA, Selim MH. The dilemma of resuming anticoagulation after intracranial hemorrhage. Stroke 2011;42(12):3665‐6. [DOI: ] - PubMed
Nielsen 2015
-
- Nielsen PB, Larsen TB, Skjøth F, Gorst‐Rasmussen A, Rasmussen LH, Lip GY. Restarting anticoagulant treatment after intracranial haemorrhage in patients with atrial fibrillation and the impact on recurrent stroke, mortality and bleeding: a nationwide cohort study. Circulation 2015; Vol. 132, issue 6:517‐25. [DOI: 10.1161/CIRCULATIONAHA.115.015735] - DOI - PubMed
O'Donnell 2016
-
- O'Donnell MJ, Chin SL, Rangarajan S, Xavier D, Liu L, Zhang H, et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case‐control study. Lancet 2016;388(10046):761‐75. [DOI: 10.1016/S0140-6736(16)30506-2] - DOI - PubMed
Paciaroni 2011
-
- Paciaroni M, Agnelli G, Venti A, Alberti A, Acciaressi M, Caso V. Efficacy and safety of anticoagulants in the prevention of venous thromboembolism in patients with acute cerebral hemorrhage: a meta‐analysis of controlled studies. Journal of Thrombosis and Haemostasis 2011;9:893‐8. [DOI: 10.1111/j.1538-7836.2011.04241.x] - DOI - PubMed
Pasquini 2014
Pennlert 2016
Poli 2014
-
- Poli D, Antonucci E, Dentali F, Erba N, Testa S, Tiraferri E, Italian Federation of Anticoagulation Clinics (FCSA). Recurrence of ICH after resumption of anticoagulation with VK antagonists: CHIRONE study. Neurology 2014;82(12):1020. [DOI: 10.1212/WNL.0000000000000245; PUBMED: 16434655] - DOI - PubMed
Poon 2014
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sandercock 2011
Steiner 2011
Steiner 2014
Tetri 2008
-
- Tetri S, Hakala J, Juvela S, Saloheimo P, Pyhtinen J, Rusanen H, et al. Safety of low‐dose subcutaneous enoxaparin for the prevention of venous thromboembolism after primary intracerebral haemorrhage. Thrombosis Research 2008;123(2):206‐12. [DOI: 10.1016/j.thromres.2008.01.018; PUBMED: 18420258] - DOI - PubMed
Viswanathan 2006
-
- Viswanathan A, Rakich SM, Engel C, Snider R, Rosand J, Greenberg SM, et al. Antiplatelet use after intracerebral hemorrhage. Neurology 2006;66(2):206. [DOI: ; PUBMED: 16434655] - PubMed
Wasay 2008
-
- Wasay M, Khan S, Zaki K, Khealani BA, Kamal A, Azam I, et al. A non‐randomized study of safety and efficacy of heparin for DVT prophylaxis in intracerebral haemorrhage. Journal of the Pakistan Medical Association 2008;58(7):362‐4. [PUBMED: 18988406] - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

