Change in Renal Function among HIV-Infected Koreans Receiving Tenofovir Disoproxil Fumarate-Backbone Antiretroviral Therapy: A 3-Year Follow-Up Study
- PMID: 28540990
- PMCID: PMC5447108
- DOI: 10.3349/ymj.2017.58.4.770
Change in Renal Function among HIV-Infected Koreans Receiving Tenofovir Disoproxil Fumarate-Backbone Antiretroviral Therapy: A 3-Year Follow-Up Study
Abstract
Purpose: Tenofovir disoproxil fumarate (TDF) is commonly prescribed as a fixed-dose, co-formulated antiretroviral drug for HIV-1 infection. The major concern of long-term TDF use is renal dysfunction. However, little is known about the long-term patterns of changes in renal function in HIV-infected Koreans receiving TDF.
Materials and methods: We prospectively followed 50 HIV-infected Koreans, performing laboratory tests every 3 months during the first year and every 6 months for the next 2 years. Urine N-acetyl-β-D-glucosaminidase (NAG) and plasma cystatin-C were measured using samples collected in the first year. Data on renal function were retrospectively collected on HIV-infected patients receiving first-line TDF (n=40) and in antiretroviral therapy (ART)-naïve patients (n=24) for 3 years. Renal function was evaluated as estimated glomerular filtration rate (eGFR) from serum creatinine [Modification of Diet in Renal Disease (MDRD)] and cystatin-C.
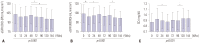
Results: The eGFR (cystatin-C) showed significant changes from 0 to 48 wks (p=0.002), with the lowest levels at 24 wks (84.3±18.8 mL/min vs. 90.3±22.5 mL/min, p=0.021 by post hoc test). Urine NAG levels did not differ at 0, 12, 24, and 48 wks, although eGFR (MDRD) significantly decreased from 0 (98.7±18.9 mL/min/1.73 m²) to 144 wks (89.0±14.7 mL/min/1.73 m²) (p=0.010). The first-line TDF group had significantly lower eGFR (MDRD) than the ART-naïve group at 144 wks (89.7 mL/min/1.73 m² vs. 98.4 mL/min/1.73 m², p=0.036). Thirteen (26%) participants experienced a decrease in renal impairment of 10 mL/min/1.73 m² in eGFR (MDRD) at 144 wks.
Conclusion: These data suggest that clinically meaningful renal injury can develop in HIV-infected Koreans receiving long-term TDF.
Keywords: HIV; anti-retroviral therapy; cystatin-C; eGFR; renal toxicity; tenofovir.
© Copyright: Yonsei University College of Medicine 2017
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures
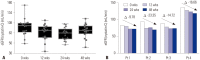

References
-
- Hemkens LG, Ewald H, Santini-Oliveira M, Bühler JE, Vuichard D, Schandelmaier S, et al. Comparative effectiveness of tenofovir in treatment-naïve HIV-infected patients: systematic review and meta-analysis. HIV Clin Trials. 2015;16:178–189. - PubMed
-
- Labhardt ND, Bader J, Lejone TI, Ringera I, Puga D, Glass TR, et al. Is zidovudine first-line therapy virologically comparable to tenofovir in resource-limited settings? Trop Med Int Health. 2015;20:914–918. - PubMed
-
- Gallant JE, Staszewski S, Pozniak AL, DeJesus E, Suleiman JM, Miller MD, et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA. 2004;292:191–201. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

