Amniotic Mesenchymal Stromal Cells Exhibit Preferential Osteogenic and Chondrogenic Differentiation and Enhanced Matrix Production Compared With Adipose Mesenchymal Stromal Cells
- PMID: 28541092
- PMCID: PMC5832055
- DOI: 10.1177/0363546517706138
Amniotic Mesenchymal Stromal Cells Exhibit Preferential Osteogenic and Chondrogenic Differentiation and Enhanced Matrix Production Compared With Adipose Mesenchymal Stromal Cells
Abstract
Background: Therapeutic efficacy of various mesenchymal stromal cell (MSC) types for orthopaedic applications is currently being investigated. While the concept of MSC therapy is well grounded in the basic science of healing and regeneration, little is known about individual MSC populations in terms of their propensity to promote the repair and/or regeneration of specific musculoskeletal tissues. Two promising MSC sources, adipose and amnion, have each demonstrated differentiation and extracellular matrix (ECM) production in the setting of musculoskeletal tissue regeneration. However, no study to date has directly compared the differentiation potential of these 2 MSC populations.
Purpose: To compare the ability of human adipose- and amnion-derived MSCs to undergo osteogenic and chondrogenic differentiation.
Study design: Controlled laboratory study.
Methods: MSC populations from the human term amnion were quantified and characterized via cell counting, histologic assessment, and flow cytometry. Differentiation of these cells in comparison to commercially purchased human adipose-derived mesenchymal stromal cells (hADSCs) in the presence and absence of differentiation media was evaluated via reverse transcription polymerase chain reaction (PCR) for bone and cartilage gene transcript markers and histology/immunohistochemistry to examine ECM production. Analysis of variance and paired t tests were performed to compare results across all cell groups investigated.
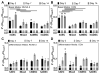
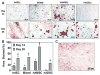
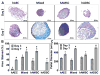
Results: The authors confirmed that the human term amnion contains 2 primary cell types demonstrating MSC characteristics-(1) human amniotic epithelial cells (hAECs) and (2) human amniotic mesenchymal stromal cells (hAMSCs)-and each exhibited more than 90% staining for MSC surface markers (CD90, CD105, CD73). Average viable hAEC and hAMSC yields at harvest were 2.3 × 106 ± 3.7 × 105 and 1.6 × 106 ± 4.7 × 105 per milliliter of amnion, respectively. As well, hAECs and hAMSCs demonstrated significantly greater osteocalcin ( P = .025), aggrecan ( P < .0001), and collagen type 2 ( P = .044) gene expression compared with hADSCs, respectively, after culture in differentiation medium. Moreover, both hAECs and hAMSCs produced significantly greater quantities of mineralized ( P < .0001) and cartilaginous ( P = .0004) matrix at earlier time points compared with hADSCs when cultured under identical osteogenic and chondrogenic differentiation conditions, respectively.
Conclusion: Amnion-derived MSCs demonstrate a greater differentiation potential toward bone and cartilage compared with hADSCs.
Clinical relevance: Amniotic MSCs may be the source of choice in the regenerative treatment of bone or osteochondral musculoskeletal disease. They show significantly higher yields and better differentiation toward these tissues than MSCs derived from adipose.
Keywords: adipose; bone; cartilage; differentiation; orthopaedics; perinatal; regenerative medicine; stromal cell.
Conflict of interest statement
The authors declared that they have no conflicts of interest in the authorship and publication of this contribution.
Figures



Similar articles
-
Human amnion-derived mesenchymal stem cells promote osteogenic and angiogenic differentiation of human adipose-derived stem cells.PLoS One. 2017 Oct 11;12(10):e0186253. doi: 10.1371/journal.pone.0186253. eCollection 2017. PLoS One. 2017. PMID: 29020045 Free PMC article.
-
Comparative characterization of cultured human term amnion epithelial and mesenchymal stromal cells for application in cell therapy.Cell Transplant. 2008;17(8):955-68. doi: 10.3727/096368908786576507. Cell Transplant. 2008. PMID: 19069637
-
Differential Effector Response of Amnion- and Adipose-Derived Mesenchymal Stem Cells to Inflammation; Implications for Intradiscal Therapy.J Orthop Res. 2019 Nov;37(11):2445-2456. doi: 10.1002/jor.24412. Epub 2019 Jul 25. J Orthop Res. 2019. PMID: 31287173
-
Characteristics and Therapeutic Potential of Human Amnion-Derived Stem Cells.Int J Mol Sci. 2021 Jan 19;22(2):970. doi: 10.3390/ijms22020970. Int J Mol Sci. 2021. PMID: 33478081 Free PMC article. Review.
-
Chondrogenesis of mesenchymal stem cells: role of tissue source and inducing factors.Stem Cell Res Ther. 2010 Oct 13;1(4):31. doi: 10.1186/scrt31. Stem Cell Res Ther. 2010. PMID: 20959030 Free PMC article. Review.
Cited by
-
Comparison of miRNA cargo in human adipose-tissue vs. amniotic-membrane derived mesenchymal stromal cells extracellular vesicles for osteoarthritis treatment.Extracell Vesicles Circ Nucl Acids. 2021 Aug 3;2(3):202-221. doi: 10.20517/evcna.2021.11. eCollection 2021. Extracell Vesicles Circ Nucl Acids. 2021. PMID: 39697592 Free PMC article.
-
Human mesenchymal stem cell treatment of premature ovarian failure: new challenges and opportunities.Stem Cell Res Ther. 2021 Mar 3;12(1):161. doi: 10.1186/s13287-021-02212-0. Stem Cell Res Ther. 2021. PMID: 33658073 Free PMC article. Review.
-
The improvement of inflammatory infiltration and pregnancy outcome in mice with recurrent spontaneous abortion by human amniotic mesenchymal stem cells.Biol Reprod. 2024 Aug 15;111(2):351-360. doi: 10.1093/biolre/ioae074. Biol Reprod. 2024. PMID: 38718142 Free PMC article.
-
Mesenchymal Stem Cells, Bioactive Factors, and Scaffolds in Bone Repair: From Research Perspectives to Clinical Practice.Cells. 2021 Jul 29;10(8):1925. doi: 10.3390/cells10081925. Cells. 2021. PMID: 34440694 Free PMC article. Review.
-
Stem cells and COVID-19: are the human amniotic cells a new hope for therapies against the SARS-CoV-2 virus?Stem Cell Res Ther. 2021 Mar 1;12(1):155. doi: 10.1186/s13287-021-02216-w. Stem Cell Res Ther. 2021. PMID: 33648582 Free PMC article. Review.
References
-
- Abumaree MH, Al Jumah MA, Kalionis B, et al. Human placental mesenchymal stem cells (pMSCs) play a role as immune suppressive cells by shifting macrophage differentiation from inflammatory M1 to anti-inflammatory M2 macrophages. Stem Cell Rev. 2013;9(5):620–641. - PubMed
-
- Barbati A, Grazia Mameli M, Sidoni A, Di Renzo GC. Amniotic membrane: separation of amniotic mesoderm from amniotic epithelium and isolation of their respective mesenchymal stromal and epithelial cells. Curr Protoc Stem Cell Biol. 2012;Chapter 1(Unit 1E.8) - PubMed
-
- Caplan AI. Mesenchymal stem cells: cell-based reconstructive therapy in orthopedics. Tiss Eng. 2005;11(7):1198–1211. - PubMed
-
- Centeno CJ, Busse D, Kisiday J, Keohan C, Freeman M, Karli D. Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells. Pain Physician. 2008;11(3):343–353. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

