Factory-Calibrated Continuous Glucose Sensors: The Science Behind the Technology
- PMID: 28541139
- PMCID: PMC5444502
- DOI: 10.1089/dia.2017.0025
Factory-Calibrated Continuous Glucose Sensors: The Science Behind the Technology
Abstract
The use of commercially available continuous glucose monitors for diabetes management requires sensor calibrations, which until recently are exclusively performed by the patient. A new development is the implementation of factory calibration for subcutaneous glucose sensors, which eliminates the need for user calibrations and the associated blood glucose tests. Factory calibration means that the calibration process is part of the sensor manufacturing process and performed under controlled laboratory conditions. The ability to move from a user calibration to factory calibration is based on several technical requirements related to sensor stability and the robustness of the sensor manufacturing process. The main advantages of factory calibration over the conventional user calibration are: (a) more convenience for the user, since no more fingersticks are required for calibration and (b) elimination of use errors related to the execution of the calibration process, which can lead to sensor inaccuracies. The FreeStyle Libre™ and FreeStyle Libre Pro™ flash continuous glucose monitoring systems are the first commercially available sensor systems using factory-calibrated sensors. For these sensor systems, no user calibrations are required throughout the sensor wear duration.
Keywords: Calibration; Continuous Glucose Monitoring; Factory calibration; Glucose sensor; Subcutaneous.
Conflict of interest statement
U.H. and E.B. are employees of Abbott Diabetes Care.
Figures
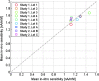

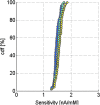
References
-
- Garg SK: Role of emerging new technologies. Diabetes Technol Ther 2008;10:413–414 - PubMed
-
- Skyler JS: CGM—a technology in evolution. Diabetes Technol Ther 2009;11:63–64 - PubMed
-
- Mastrototaro JJ: The MiniMed continuous glucose monitoring system. Diabetes Technol Ther 2000;2 Suppl 1:S13–S18 - PubMed
-
- Reach G, Wilson GS: Can continuous glucose monitoring be used for the treatment of diabetes. Anal Chem 1992;64:381A–386A - PubMed
-
- Gough DA, Armour JC, Baker DA: Advances and prospects in glucose assay technology. Diabetologia 1997;40:S102–S107 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
