Hyperferritinemia and inflammation
- PMID: 28541437
- PMCID: PMC5890889
- DOI: 10.1093/intimm/dxx031
Hyperferritinemia and inflammation
Abstract
Understanding of ferritin biology has traditionally centered on its role in iron storage and homeostasis, with low ferritin levels indicative of deficiency and high levels indicative of primary or secondary hemochromatosis. However, further work has shown that iron, redox biology and inflammation are inexorably linked. During infection, increased ferritin levels represent an important host defense mechanism that deprives bacterial growth of iron and protects immune cell function. It may also be protective, limiting the production of free radicals and mediating immunomodulation. Additionally, hyperferritinemia is a key acute-phase reactants, used by clinicians as an indication for therapeutic intervention, aimed at controlling inflammation in high-risk patients. One school of thought maintains that hyperferritinemia is an 'innocent bystander' biomarker of uncontrolled inflammation that can be used to gauge effectiveness of intervention. Other schools of thought maintain that ferritin induction could be a protective negative regulatory loop. Others maintain that ferritin is a key mediator of immune dysregulation, especially in extreme hyperferritinemia, via direct immune-suppressive and pro-inflammatory effects. There is a clear need for further investigation of the role of ferritin in uncontrolled inflammatory conditions both as a biomarker and mediator of disease because its occurrence identifies patients with high mortality risk and its resolution predicts their improved survival.
Keywords: ferritin; hemophagocytic lymphohistiocytosis; hemophagocytosis; iron; macrophage activation.
© The Japanese Society for Immunology. 2017. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
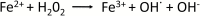


References
-
- Cricthon R. R. and Chaloteaux-Wauters M. 1987. Iron transport and storage. Eur. J. Biochem. 164:485. - PubMed
-
- Cohen L. A., Gutierrez L., Weiss A. et al. . 2010. Serum ferritin is derived primarily from macrophages through a nonclassical secretory pathway. Blood 116:1574. - PubMed
-
- Kato J., Kobune M., Ohkubo S. et al. . 2007. Iron/IRP-1-dependent regulation of mRNA expression for transferrin receptor, DMT1 and ferritin during human erythroid differentiation. Exp. Hematol. 35:879. - PubMed
-
- Harrison P. M. and Arosio P. 1996. The ferritins: molecular properties, iron storage function and cellular regulation. Biochim. Biophys. Acta 1275:161. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

