The Evolutionary Landscape of Dbl-Like RhoGEF Families: Adapting Eukaryotic Cells to Environmental Signals
- PMID: 28541439
- PMCID: PMC5499878
- DOI: 10.1093/gbe/evx100
The Evolutionary Landscape of Dbl-Like RhoGEF Families: Adapting Eukaryotic Cells to Environmental Signals
Abstract
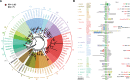
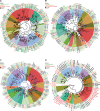
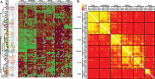
The dynamics of cell morphology in eukaryotes is largely controlled by small GTPases of the Rho family. Rho GTPases are activated by guanine nucleotide exchange factors (RhoGEFs), of which diffuse B-cell lymphoma (Dbl)-like members form the largest family. Here, we surveyed Dbl-like sequences from 175 eukaryotic genomes and illuminate how the Dbl family evolved in all eukaryotic supergroups. By combining probabilistic phylogenetic approaches and functional domain analysis, we show that the human Dbl-like family is made of 71 members, structured into 20 subfamilies. The 71 members were already present in ancestral jawed vertebrates, but several members were subsequently lost in specific clades, up to 12% in birds. The jawed vertebrate repertoire was established from two rounds of duplications that occurred between tunicates, cyclostomes, and jawed vertebrates. Duplicated members showed distinct tissue distributions, conserved at least in Amniotes. All 20 subfamilies have members in Deuterostomes and Protostomes. Nineteen subfamilies are present in Porifera, the first phylum that diverged in Metazoa, 14 in Choanoflagellida and Filasterea, single-celled organisms closely related to Metazoa and three in Fungi, the sister clade to Metazoa. Other eukaryotic supergroups show an extraordinary variability of Dbl-like repertoires as a result of repeated and independent gain and loss events. Last, we observed that in Metazoa, the number of Dbl-like RhoGEFs varies in proportion of cell signaling complexity. Overall, our analysis supports the conclusion that Dbl-like RhoGEFs were present at the origin of eukaryotes and evolved as highly adaptive cell signaling mediators.
Keywords: Dbl; Rho GTPases; cell signaling; guanine nucleotide exchange factors.
© The Author 2017. Published by Oxford University Press on behalf of the Society for Molecular Biology and Evolution.
Figures




Similar articles
-
Evolution of the Rho family of ras-like GTPases in eukaryotes.Mol Biol Evol. 2007 Jan;24(1):203-16. doi: 10.1093/molbev/msl145. Epub 2006 Oct 11. Mol Biol Evol. 2007. PMID: 17035353 Free PMC article.
-
Dbl family RhoGEFs in cancer: different roles and targeting strategies.Biochem Pharmacol. 2024 May;223:116141. doi: 10.1016/j.bcp.2024.116141. Epub 2024 Mar 16. Biochem Pharmacol. 2024. PMID: 38499108 Review.
-
Targeting the Dbl and dock-family RhoGEFs: a yeast-based assay to identify cell-active inhibitors of Rho-controlled pathways.Enzymes. 2013;33 Pt A:169-91. doi: 10.1016/B978-0-12-416749-0.00008-7. Epub 2013 Aug 8. Enzymes. 2013. PMID: 25033805 Review.
-
Phylogeny of the CDC25 homology domain reveals rapid differentiation of Ras pathways between early animals and fungi.Cell Signal. 2009 Nov;21(11):1579-85. doi: 10.1016/j.cellsig.2009.06.004. Epub 2009 Jun 28. Cell Signal. 2009. PMID: 19567266
-
Rho guanine nucleotide exchange factors: regulators of Rho GTPase activity in development and disease.Oncogene. 2014 Jul 31;33(31):4021-35. doi: 10.1038/onc.2013.362. Epub 2013 Sep 16. Oncogene. 2014. PMID: 24037532 Free PMC article. Review.
Cited by
-
Regulation of the Actin Cytoskeleton via Rho GTPase Signalling in Dictyostelium and Mammalian Cells: A Parallel Slalom.Cells. 2021 Jun 24;10(7):1592. doi: 10.3390/cells10071592. Cells. 2021. PMID: 34202767 Free PMC article. Review.
-
The potential therapeutic roles of Rho GTPases in substance dependence.Front Mol Neurosci. 2023 Mar 30;16:1125277. doi: 10.3389/fnmol.2023.1125277. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37063367 Free PMC article.
-
Rho GTPases in Skeletal Muscle Development and Homeostasis.Cells. 2021 Nov 2;10(11):2984. doi: 10.3390/cells10112984. Cells. 2021. PMID: 34831205 Free PMC article. Review.
-
Rho GTPases in Gynecologic Cancers: In-Depth Analysis toward the Paradigm Change from Reactive to Predictive, Preventive, and Personalized Medical Approach Benefiting the Patient and Healthcare.Cancers (Basel). 2020 May 20;12(5):1292. doi: 10.3390/cancers12051292. Cancers (Basel). 2020. PMID: 32443784 Free PMC article. Review.
-
Role of Rho-associated kinases and their inhibitor fasudil in neurodegenerative diseases.Front Neurosci. 2024 Nov 19;18:1481983. doi: 10.3389/fnins.2024.1481983. eCollection 2024. Front Neurosci. 2024. PMID: 39628659 Free PMC article. Review.
References
-
- Abascal F, Zardoya R, Posada D.. 2005. ProtTest: selection of best-fit models of protein evolution. Bioinformatics 21:2104–2105. - PubMed
-
- Ashkin A, Schütze K, Dziedzic JM, Euteneuer U, Schliwa M.. 1990. Force generation of organelle transport measured in vivo by an infrared laser trap. Nature. 348:346–348. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

