Necessity of Bumped Kinase Inhibitor Gastrointestinal Exposure in Treating Cryptosporidium Infection
- PMID: 28541457
- PMCID: PMC5853285
- DOI: 10.1093/infdis/jix247
Necessity of Bumped Kinase Inhibitor Gastrointestinal Exposure in Treating Cryptosporidium Infection
Abstract
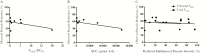
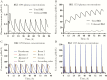
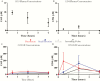
There is a substantial need for novel therapeutics to combat the widespread impact caused by Crytosporidium infection. However, there is a lack of knowledge as to which drug pharmacokinetic (PK) characteristics are key to generate an in vivo response, specifically whether systemic drug exposure is crucial for in vivo efficacy. To identify which PK properties are correlated with in vivo efficacy, we generated physiologically based PK models to simulate systemic and gastrointestinal drug concentrations for a series of bumped kinase inhibitors (BKIs) that have nearly identical in vitro potency against Cryptosporidium but display divergent PK properties. When BKI concentrations were used to predict in vivo efficacy with a neonatal model of Cryptosporidium infection, these concentrations in the large intestine were the sole predictors of the observed in vivo efficacy. The significance of large intestinal BKI exposure for predicting in vivo efficacy was further supported with an adult mouse model of Cryptosporidium infection. This study suggests that drug exposure in the large intestine is essential for generating a superior in vivo response, and that physiologically based PK models can assist in the prioritization of leading preclinical drug candidates for in vivo testing.
Keywords: Cryptosporidium; drug development; gastrointestinal; physiologically based pharmacokinetic model.
© The Author 2017. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures



References
-
- Kotloff KL, Nataro JP, Blackwelder WC, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 2013; 382:209–22. - PubMed
-
- Amadi B, Mwiya M, Musuku J, et al. Effect of nitazoxanide on morbidity and mortality in Zambian children with cryptosporidiosis: a randomised controlled trial. Lancet 2002; 360:1375–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

