Mitochondrial Retroprocessing Promoted Functional Transfers of rpl5 to the Nucleus in Grasses
- PMID: 28541477
- PMCID: PMC5850859
- DOI: 10.1093/molbev/msx170
Mitochondrial Retroprocessing Promoted Functional Transfers of rpl5 to the Nucleus in Grasses
Abstract
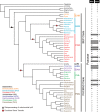
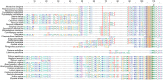
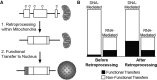
Functional gene transfers from the mitochondrion to the nucleus are ongoing in angiosperms and have occurred repeatedly for all 15 ribosomal protein genes, but it is not clear why some of these genes are transferred more often than others nor what the balance is between DNA- and RNA-mediated transfers. Although direct insertion of mitochondrial DNA into the nucleus occurs frequently in angiosperms, case studies of functional mitochondrial gene transfer have implicated an RNA-mediated mechanism that eliminates introns and RNA editing sites, which would otherwise impede proper expression of mitochondrial genes in the nucleus. To elucidate the mechanisms that facilitate functional gene transfers and the evolutionary dynamics of the coexisting nuclear and mitochondrial gene copies that are established during these transfers, we have analyzed rpl5 genes from 90 grasses (Poaceae) and related monocots. Multiple lines of evidence indicate that rpl5 has been functionally transferred to the nucleus at least three separate times in the grass family and that at least seven species have intact and transcribed (but not necessarily functional) copies in both the mitochondrion and nucleus. In two grasses, likely functional nuclear copies of rpl5 have been subject to recent gene conversion events via secondarily transferred mitochondrial copies in what we believe are the first described cases of mitochondrial-to-nuclear gene conversion. We show that rpl5 underwent a retroprocessing event within the mitochondrial genome early in the evolution of the grass family, which we argue predisposed the gene towards successful, DNA-mediated functional transfer by generating a "pre-edited" sequence.
Keywords: endosymbiotic gene transfer; intracellular gene transfer; mtDNA; pseudogene; reverse transcription.
© The Author 2017. Published by Oxford University Press on behalf of the Society for Molecular Biology and Evolution. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures



Similar articles
-
Pervasive survival of expressed mitochondrial rps14 pseudogenes in grasses and their relatives for 80 million years following three functional transfers to the nucleus.BMC Evol Biol. 2006 Jul 14;6:55. doi: 10.1186/1471-2148-6-55. BMC Evol Biol. 2006. PMID: 16842621 Free PMC article.
-
Transfer of RPS14 and RPL5 from the mitochondrion to the nucleus in grasses.Gene. 2004 Jan 7;324:139-47. doi: 10.1016/j.gene.2003.09.027. Gene. 2004. PMID: 14693379
-
Repeated, recent and diverse transfers of a mitochondrial gene to the nucleus in flowering plants.Nature. 2000 Nov 16;408(6810):354-7. doi: 10.1038/35042567. Nature. 2000. PMID: 11099041
-
Evolution of mitochondrial gene content: gene loss and transfer to the nucleus.Mol Phylogenet Evol. 2003 Dec;29(3):380-95. doi: 10.1016/s1055-7903(03)00194-5. Mol Phylogenet Evol. 2003. PMID: 14615181 Review.
-
Mitochondrial RNA maturation.RNA Biol. 2024 Jan;21(1):28-39. doi: 10.1080/15476286.2024.2414157. Epub 2024 Oct 10. RNA Biol. 2024. PMID: 39385590 Free PMC article. Review.
Cited by
-
Complete mitochondrial genome of the endangered Prunus pedunculata (Prunoideae, Rosaceae) in China: characterization and phylogenetic analysis.Front Plant Sci. 2023 Dec 8;14:1266797. doi: 10.3389/fpls.2023.1266797. eCollection 2023. Front Plant Sci. 2023. PMID: 38155854 Free PMC article.
-
Recombination and retroprocessing in broomrapes reveal a universal roadmap for mitochondrial evolution in heterotrophic plants.bioRxiv [Preprint]. 2025 Feb 16:2025.02.14.637881. doi: 10.1101/2025.02.14.637881. bioRxiv. 2025. PMID: 39990427 Free PMC article. Preprint.
-
Chloroplast genomes in Populus (Salicaceae): comparisons from an intensively sampled genus reveal dynamic patterns of evolution.Sci Rep. 2021 May 4;11(1):9471. doi: 10.1038/s41598-021-88160-4. Sci Rep. 2021. PMID: 33947883 Free PMC article.
-
Rewiring of Aminoacyl-tRNA Synthetase Localization and Interactions in Plants With Extensive Mitochondrial tRNA Gene Loss.Mol Biol Evol. 2023 Jul 5;40(7):msad163. doi: 10.1093/molbev/msad163. Mol Biol Evol. 2023. PMID: 37463427 Free PMC article.
-
Continuous infiltration and evolutionary trajectory of nuclear organelle DNA in Oryza.Genome Res. 2025 Jun 2;35(6):1349-1363. doi: 10.1101/gr.279609.124. Genome Res. 2025. PMID: 40280772
References
-
- Adams KL, Daley DO, Qiu YL, Whelan J, Palmer JD.. 2000. Repeated, recent and diverse transfers of a mitochondrial gene to the nucleus in flowering plants. Nature 408:354–357. - PubMed
-
- Adams KL, Palmer JD.. 2003. Evolution of mitochondrial gene content: gene loss and transfer to the nucleus. Mol Phylogenet Evol. 29:380–395. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

