Comparison of first-line chemotherapy including escalated BEACOPP versus chemotherapy including ABVD for people with early unfavourable or advanced stage Hodgkin lymphoma
- PMID: 28541603
- PMCID: PMC6481581
- DOI: 10.1002/14651858.CD007941.pub3
Comparison of first-line chemotherapy including escalated BEACOPP versus chemotherapy including ABVD for people with early unfavourable or advanced stage Hodgkin lymphoma
Abstract
Background: There are two different international standards for the treatment of early unfavourable and advanced stage Hodgkin lymphoma (HL): chemotherapy with escalated BEACOPP (bleomycin/etoposide/doxorubicin/cyclophosphamide/vincristine/procarbazine/prednisone) regimen and chemotherapy with ABVD (doxorubicin/bleomycin/vinblastine/dacarbazine) regimen.
Objectives: To determine the advantages and disadvantages of chemotherapy including escalated BEACOPP compared to chemotherapy including ABVD in the treatment of early unfavourable or advanced stage HL as first-line treatment.
Search methods: We searched for randomised controlled trials in MEDLINE, CENTRAL and conference proceedings (January 1985 to July 2013 and for the update to March 2017) and Embase (1985 to November 2008). Moreover we searched trial registries (March 2017; www.controlled-trials.com, www.clinicaltrialsregister.eu/ctr-search/search, clinicaltrials.gov, www.eortc.be, www.ghsg.org, www.ctc.usyd.edu.au, www.trialscentral.org/index.html) SELECTION CRITERIA: We included randomised controlled trials examining chemotherapy including at least two cycles of escalated BEACOPP regimens compared with chemotherapy including at least four cycles of ABVD regimens as first-line treatment for patients with early unfavourable stage or advanced stage HL.
Data collection and analysis: The effect measures we used were hazard ratios (HRs) for overall survival (OS), progression-free survival (PFS) and freedom from first progression.We used risk ratios (RRs) relative risks to analyse harms: treatment-related mortality, secondary malignancies (including myeloid dysplastic syndrome (MDS) or acute myeloid leukaemia (AML)), infertility and adverse events.Quality of life was not reported in any trial, therefore not analysed. Two review authors independently extracted data and assessed quality of trials.
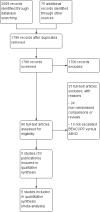
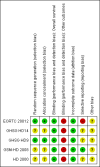
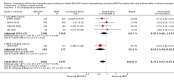
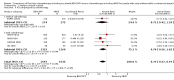
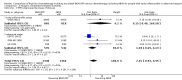
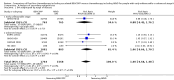
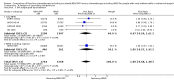
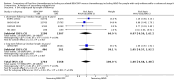
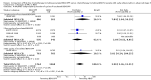
Main results: We screened 1796 records and identified five eligible trials in total i.e. one trial could be added on the previous review. These trials included only adults (16 to 65 years of age). We included all five trials with 3427 people in the meta-analyses: the HD9 and HD14 trials were co-ordinated in Germany, the HD2000 and GSM-HD trials were performed in Italy and the EORTC 20012 was conducted in Belgium. The overall risk of performance and detection bias was low for overall survival (OS), but was high for other outcomes, as therapy blinding was not feasible. The remaining 'Risk of bias' domains were low and unclear.All trials reported results for OS and progression-free survival (PFS). In contrast to the our first published review (2011) the addition of results from the EORTC 20012 BEACOPP escalated increases OS (3142 participants; HR 0.74 (95% confidence interval (CI) 0.57 to 0.97; high-quality evidence). This means that only 90 (70 to 117) patients will die after five years in the BEACOPP escalated arm compared to 120 in the ABVD arm. This survival advantage is also reflected in an increased PFS with BEACOPP escalated (3142 participants; HR 0.54 (95% CI 0.45 to 0.64); moderate-quality evidence), meaning that after five years only 144 (121 to 168) patients will experience a progress, relapse or death in the BEACOPP escalated arm compared to 250 in the ABVD arm.There is no evidence for a difference for treatment-related mortality (2700 participants, RR 2.15 (95% CI = 0.93 to 4.95), low-quality evidence).Although the occurrence of MDS or AML may increase with BEACOPP escalated (3332 participants, RR 3.90 (95% CI 1.36 to 11.21); low-quality evidence)), there is no evidence for a difference between both regimens for overall secondary malignancies (3332 participants, RR 1.00 (95% CI 0.68 to 1.48), low-quality evidence). However, the observation time of the studies included in the review is too short to be expected to demonstrate differences with respect to second solid tumours which would not be expected to show significance until around 15 years after treatment.We are very uncertain how many female patients will be infertile due to chemotherapy and which arm might be favoured (106 participants, RR 1.37 (95% CI 0.83 to 2.26), very low-quality evidence). This is a very small sample, and the age of the patients was not detailed. No analysis of male fertility was provided.Five trials reported adverse events and the analysis shows that the escalated BEACOPP regimens probably causes more haematological toxicities WHO grade III or IV ((anaemia: 2425 participants, RR 10.67 (95% CI 7.14 to 15.93); neutropenia: 519 participants, RR 1.80 (95% CI 1.52 to 2.13); thrombocytopenia: 2425 participants, RR 18.12 (95% CI 11.77 to 27.92); infections: 2425 participants, RR 3.73 (95% CI 2.58 to 5.38), all low-quality evidence).Only one trial (EORTC 20012) planned to assess quality of life, however, no results were reported.
Authors' conclusions: This meta-analysis provides moderate- to high-quality evidence that adult patients between 16 and 60 years of age with early unfavourable and advanced stage HL benefit regarding OS and PFS from first-line chemotherapy including escalated BEACOPP. The proven benefit in OS for patients with advanced HL is a new finding of this updated review due to the inclusion of the results from the EORTC 20012 trial. Furthermore, there is only low-quality evidence of a difference in the total number of secondary malignancies, as the follow-up period might be too short to detect meaningful differences. Low-quality evidence also suggests that people treated with escalated BEACOPP may have a higher risk to develop secondary AML or MDS. Due to the availability of only very low-quality evidence available, we are unable to come to a conclusion in terms of infertility. This review does for the first time suggest a survival benefit. However, it is clear from this review that BEACOPP escalated may be more toxic that ABVD, and very important long-term side effects of second malignancies and infertility have not been sufficiently analysed yet.
Conflict of interest statement
AW: none known
IM: none known
CB: none known
AE is chairman of the German Hodgkin Study Group and conducted two of the five included studies (GHSG HD9; GHSG HD14).
BT is study physician of the GHSG and was sub‐investigator in GHSG HD14.
NS: none known
Figures









































Update of
-
Comparison of chemotherapy including escalated BEACOPP versus chemotherapy including ABVD for patients with early unfavourable or advanced stage Hodgkin lymphoma.Cochrane Database Syst Rev. 2011 Aug 10;(8):CD007941. doi: 10.1002/14651858.CD007941.pub2. Cochrane Database Syst Rev. 2011. Update in: Cochrane Database Syst Rev. 2017 May 25;5:CD007941. doi: 10.1002/14651858.CD007941.pub3. PMID: 21833963 Updated.
Similar articles
-
Comparison of chemotherapy including escalated BEACOPP versus chemotherapy including ABVD for patients with early unfavourable or advanced stage Hodgkin lymphoma.Cochrane Database Syst Rev. 2011 Aug 10;(8):CD007941. doi: 10.1002/14651858.CD007941.pub2. Cochrane Database Syst Rev. 2011. Update in: Cochrane Database Syst Rev. 2017 May 25;5:CD007941. doi: 10.1002/14651858.CD007941.pub3. PMID: 21833963 Updated.
-
Optimisation of chemotherapy and radiotherapy for untreated Hodgkin lymphoma patients with respect to second malignant neoplasms, overall and progression-free survival: individual participant data analysis.Cochrane Database Syst Rev. 2017 Sep 13;9(9):CD008814. doi: 10.1002/14651858.CD008814.pub2. Cochrane Database Syst Rev. 2017. PMID: 28901021 Free PMC article.
-
Positron emission tomography-adapted therapy for first-line treatment in adults with Hodgkin lymphoma.Cochrane Database Syst Rev. 2025 Mar 26;3(3):CD010533. doi: 10.1002/14651858.CD010533.pub3. Cochrane Database Syst Rev. 2025. PMID: 40135712
-
Positron emission tomography-adapted therapy for first-line treatment in individuals with Hodgkin lymphoma.Cochrane Database Syst Rev. 2015 Jan 9;1(1):CD010533. doi: 10.1002/14651858.CD010533.pub2. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2025 Mar 26;3:CD010533. doi: 10.1002/14651858.CD010533.pub3. PMID: 25572491 Free PMC article. Updated.
-
Chemotherapy alone versus chemotherapy plus radiotherapy for adults with early stage Hodgkin lymphoma.Cochrane Database Syst Rev. 2017 Apr 27;4(4):CD007110. doi: 10.1002/14651858.CD007110.pub3. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2024 Dec 2;12:CD007110. doi: 10.1002/14651858.CD007110.pub4. PMID: 28447341 Free PMC article. Updated.
Cited by
-
Survival rates of adult patients with Hodgkin lymphoma who underwent ABVD versus escalated BEACOPP in a resource-limited country: An observational study.Cancer Rep (Hoboken). 2023 Aug;6(8):e1839. doi: 10.1002/cnr2.1839. Epub 2023 May 31. Cancer Rep (Hoboken). 2023. PMID: 37254799 Free PMC article.
-
Viral oncogenesis in cancer: from mechanisms to therapeutics.Signal Transduct Target Ther. 2025 May 12;10(1):151. doi: 10.1038/s41392-025-02197-9. Signal Transduct Target Ther. 2025. PMID: 40350456 Free PMC article. Review.
-
AYA Considerations for Aggressive Lymphomas.Curr Hematol Malig Rep. 2021 Feb;16(1):61-71. doi: 10.1007/s11899-021-00607-7. Epub 2021 Mar 16. Curr Hematol Malig Rep. 2021. PMID: 33728589 Review.
-
Hodgkin Lymphoma-Review on Pathogenesis, Diagnosis, Current and Future Treatment Approaches for Adult Patients.J Clin Med. 2021 Mar 8;10(5):1125. doi: 10.3390/jcm10051125. J Clin Med. 2021. PMID: 33800409 Free PMC article. Review.
-
How I Follow Hodgkin Lymphoma in First Complete (Metabolic) Remission?Medicina (Kaunas). 2024 Feb 19;60(2):344. doi: 10.3390/medicina60020344. Medicina (Kaunas). 2024. PMID: 38399631 Free PMC article. Review.
References
References to studies included in this review
EORTC 20012 {published data only}
-
- Carde P, Karrash M, Fortpied C, Brice P, Khaled H, Casasnovas O, et al. Eight cycles of ABVD versus four cycles of BEACOPP escalated plus four cycles of BEACOPP baseline in Stage III to IV, International Prognostic Score ≥ 3, high‐risk Hodgkin lymphoma: First results of the Phase III EORTC 20012 Intergroup Trial. Journal of Clinical Oncology June 2016;34(17):2028‐36. - PubMed
-
- Carde PP, Karrasch M, Fortpied C, Brice P, Khaled HM, Caillot D, et al. ABVD (8 cycles) versus BEACOPP (4 escalated cycles => 4 baseline) in stage III‐IV high‐risk Hodgkin lymphoma (HL): First results of EORTC 20012 Intergroup randomized phase III clinical trial. Journal of Clinical Oncology, 30, (suppl; Abstract 8002). 2012.
-
- Carde PP, Linch DC, Divine M, Sureda A, Meyer RM, Ma D, et al. Comparison of two combination chemotherapy regimens in treating patients with stage III or stage IV Hodgkin's Lymphoma, (NCT00049595). http://clinicaltrials.gov/ct2/show/NCT00049595 2010.
-
- Mounier N, Brice P, Bologna S, Briere J, Gaillard I, Heczko M, et al. ABVD (8 cycles) versus BEACOPP (4 escalated cycles ≥4 baseline): final results in stage III–IV low‐risk Hodgkin lymphoma (IPS 0–2) of the LYSA H34 randomized trial. Annals of Oncology May 2014, issue 25:1622‐8. - PubMed
GHSG HD14 {published data only}
-
- Behringer K, Mueller H, Goergen H, Thielen I, Eibl AD, Stumpf V, et al. Gonadal function and fertility in survivors after Hodgkin lymphoma treatment within the German Hodgkin Study Group HD13 to HD15 trials. Journal of Clinical Oncology 2013;31(2):231‐9. - PubMed
-
- Behringer K, Thielen I, Mueller H, Goergen H, Eibl AD, Rosenbrock J, et al. Fertility and gonadal function in female survivors after treatment of early unfavorable Hodgkin lymphoma (HL) within the German Hodgkin Study Group HD14 trial. Annals of Oncology 2012;23(7):1818‐25. - PubMed
-
- Borchmann P, Engert A, Pluetschow A, Fuchs M, Markova J, Lohri A, et al. Dose‐intensified combined modality treatment with 2 cycles of BEACOPP escalated followed by 2 cycles of ABVD and involved field radiotherapy (IF‐RT) is superior to 4 cycles of ABVD and IFRT in patients with early unfavourable Hodgkin Lymphoma (HL): An analysis of the German Hodgkin Study Group (GHSG) HD14 Trial. Blood. 2008; Vol. 112:367.
-
- Engert A, Borchmann P, Pluetschow A, Fuchs M, Markova J, Lohri A, et al. Dose‐intensified combined modality treatment with 2 cycles of BEACOPP escalated followed by cycles of ABVD and involved field radiotherapy (IF‐RT) is superior to 4 cycles of ABVD and IF‐RT in patients with early unfavourable Hodgkin lymphoma (HL): An analysis of the German Hodgkin Study Group (GHSG). Haematologica. 2009.
-
- Engert A, Borchmann P, Pluetschow A, Tresckow B, Markova J, Hitz F, et al. Dose‐escalation with BEACOPP escalated is superior to ABVD in the combined‐modality treatment of early unfavourable Hodgkin lymphoma: Final analysis of the German Hodgkin Study Group (GHSG) HD14 trial. Blood. 2010.
GHSG HD9 {published data only}
-
- Erratum: Standard and increased‐dose BEACOPP chemotherapy compared with COPP‐ABVD for advanced Hodgkin's disease (New England Journal of Medicine (June 12, 2003) 348 (2386‐2395)). New England Journal of Medicine 2005; Vol. 353, issue 7:744. - PubMed
-
- Behringer K, Breuer K, Reineke T, May M, Nogova L, Klimm B, et al. Secondary amenorrhea after Hodgkin's lymphoma is influenced by age at treatment, stage of disease, chemotherapy regimen, and the use of oral contraceptives during therapy: a report from the German Hodgkin's Lymphoma Study Group. Journal of Clinical Oncology 2005;23(30):7555‐64. - PubMed
-
- Diehl V, Brillant C, Franklin J, Engert A, Pfistner B, Greil R, et al. Optimisation of BEACOPP for advanced Hodgkin's disease: Further results from German Hodgkin Study Group trials HD9 and HD12. Annals of Oncology. 2005; Vol. 16 Suppl 5, issue 12:51.
-
- Diehl V, Brillant C, Franklin J, Hermann R, Greil R, Engert A, et al. BEACOPP chemotherapy for advanced Hodgkin's disease: Results of further analyses of the HD9‐ and HD12‐ trials of the German Hodgkin Study Group (GHSG). Blood. 2004; Vol. 104, issue 11 Part 1:91‐2.
-
- Diehl V, Brillant C, Franklin J, Hermann R, Greil R, Engert A, et al. BEACOPP chemotherapy for advanced Hodgkin's disease: results of further analyses of the HD9‐ and HD12‐ trials of the German Hodgkin Study Group (GHSG). Blood 2004;104(11):307.
GSM‐HD 2008 {published data only}
-
- Fondazione Michelangelo. Study Comparing Doxorubicin, Bleomycin,Vinblastine, Dacarbazine (ABVD) vs Bleomycin, Etoposide, Doxorubicin, Cyclophosphamide, Vincristine, Procarbazine and Prednisone (BEACOPP), ( NCT01251107). http://www.clinicaltrial.gov/ct2/show/NCT01251107 2010.
-
- Gianni AM, Rambaldi A, Zinzani P, Levis A, Brusamolino E, Pulsoni A, et al. Comparable 3‐year outcome following ABVD or BEACOPP first‐line chemotherapy, plus pre‐planned high‐dose salvage, in advanced Hodgkin lymphoma (HL): A randomized trial of the Michelangelo, GITIL and IIL cooperative groups [abstract no. 8506]. Journal of Clinical Oncology. 2008; Vol. 26, issue 15S part I:455.
-
- Viviani S, Zinzani PL, Rambaldi A, Brusamolino E, Levis A, Bonfante V, et al. ABVD versus BEACOPP for Hodgkin's lymphoma when high‐dose salvage is planned. New England Journal of Medicine 2011;365(3):203‐12. - PubMed
HD 2000 {published data only}
-
- Federico M, Luminari S, Iannitto E, Polimeno G, Marcheselli L, Montanini A, et al. ABVD compared with BEACOPP compared with CEC for the initial treatment of patients with advanced Hodgkin's lymphoma: results from the HD2000 Gruppo Italiano per lo Studio dei Linfomi Trial. Journal of Clinical Oncology 2009;27:805‐11. - PubMed
-
- Federico M, Mailander V, Dell'Olio M, Merli F, Brugiatelli M, Stelitano C, et al. ABVD vs. COPPEBVCAD (CEC) vs. BEACOPP for the initial treatment of patients with advanced Hodgkin Lymphoma (HL). Preliminary results of HD2000 GISL trial. Haematologica. 2007; Vol. 92:34.
-
- Gobbi PG, Rosti V, Valentino F, Bonetti E, Merli F, Stelitano C, et al. The early‐ and intermediate‐term toxicity to primitive hematopoietic progenitor cells of three chemotherapy regimens for advanced Hodgkin lymphoma. Clinical Lymphoma & Myeloma 2009;9:425‐9. - PubMed
-
- Gobbi PG, Valentino F, Danova M, Morabito F, Rovati B, Mammi C, et al. Bone marrow stem cell damage after three different chemotherapy regimens for advanced Hodgkin's lymphoma. Oncology Reports 2009;21:1029‐35. - PubMed
-
- Gruppo Italiano Studio Linfomi. Treatment of Advanced Hodgkin's Disease (Stages IIB‐III‐IV) (HD2000), ( NCT00443677). http://www.clinicaltrial.gov/ct2/show/NCT00443677 2007.
References to studies excluded from this review
Ballova 2005 {published data only}
-
- Ballova V, Ruffer JU, Haverkamp H, Pfistner B, Muller‐Hermelink HK, Duhmke E, et al. A prospectively randomized trial carried out by the German Hodgkin Study Group (GHSG) for elderly patients with advanced Hodgkin's disease comparing BEACOPP baseline and COPP‐ABVD (study HD9elderly). Annals of Oncology 2005;16(1):124‐31. - PubMed
Biasoli 2008 {published data only}
-
- Biasoli I, Franchi‐Rezgui P, Sibon D, Briere J, Kerviler E, Thieblemont C, et al. Analysis of factors influencing inclusion of 102 patients with stage III/IV Hodgkin's lymphoma in a randomized trial for first‐line chemotherapy. Annals of Oncology 2008;19(11):1915‐20. - PubMed
Borchmann 2011 {published data only}
-
- Borchmann P, Haverkamp H, Diehl V, Cerny T, Markova J, Ho AD, et al. Eight cycles of escalated‐dose BEACOPP compared with four cycles of escalated‐dose BEACOPP followed by four cycles of baseline‐dose BEACOPP with or without radiotherapy in patients with advanced‐stage hodgkin's lymphoma: final analysis of the HD12 trial of the German Hodgkin Study Group. Journal of Clinical Oncology 2011;29:4234‐42. - PubMed
Borchmann 2017 {published data only}
-
- Borchmann P, Haverkamp H, Lohri A, Mey U, Kreissl S, Greil R, et al. Progression‐free survival of early interim PET‐positive patients with advanced stage Hodgkin's lymphoma treated with BEACOPP escalated alone or in combination with rituximab (HD18): an open‐label, international, randomised phase 3 study by the German Hodgkin Study Group. Lancet Oncology 2017;18(4):454–63. [PUBMED: 28236583] - PubMed
Canellos 2005 {published data only}
-
- Canellos GP. Clinical trials in North America. European Journal of Haematology, Supplement 2005;75(66):121‐4. - PubMed
Cheson 2007 {published data only}
-
- Cheson BD. Is BEACOPP better than ABVD?. Current Hematologic Malignancy Reports 2007;2(3):161‐6. - PubMed
Connors 2002 {published data only}
-
- Connors JM. Current clinical trials for advanced Hodgkin's lymphoma in North America: History, design and rationale. Annals of Oncology 2002;13(Suppl 1):92‐5. - PubMed
Dann 2012 {published data only}
-
- Dann EJ, Blumenfeld Z, Bar‐Shalom R, Avivi I, Ben‐Shachar M, Goor O, et al. A 10‐year experience with treatment of high and standard risk Hodgkin disease: six cycles of tailored BEACOPP, with interim scintigraphy, are effective and female fertility is preserved. American Journal of Hematology 2012;87:32‐6. - PubMed
DeVita 2003 {published data only}
-
- DeVita VT Jr. Hodgkin's disease ‐ Clinical trials and travails. New England Journal of Medicine 2003;348(24):2375‐6. - PubMed
Diehl 1997 {published data only}
-
- Diehl V, Sieber M, Ruffler U, Lathan B, Hasenclever D, Pfreundschuh M, et al. BEACOPP: an intensified chemotherapy regimen in advanced Hodgkin's disease. The German Hodgkin's Lymphoma Study Group. Annals of Oncology 1997;8(2):143‐8. - PubMed
Economopoulos 2003 {published data only}
-
- Economopoulos T, Fountzilas G, Dimopoulos MA, Papageorgiou S, Xiros N, Kalantzis D, et al. Treatment of intermediate and advanced stage Hodgkin's disease with modified baseline BEACOPP regimen: a Hellenic Co‐operative Oncology Group Study. European Journal of Haematology 2003;71(4):257‐62. - PubMed
Eghbali 2005 {published data only}
-
- Eghbali H, Raemaekers J, Carde P. The EORTC strategy in the treatment of Hodgkin's lymphoma. European Journal of Haematology. Supplementum 2005;75(66):135‐40. - PubMed
Eich 2004 {published data only}
-
- Eich HT, Staar S, Gossmann A, Hansemann K, Skripnitchenko R, Kocher M, et al. Centralized radiation oncologic review of cross‐sectional imaging of Hodgkin's disease leads to significant changes in required involved field‐results of a quality assurance program of the German Hodgkin Study Group. International Journal of Radiation Oncology, Biology, Physics 2004;58(4):1121‐7. - PubMed
Elbl 2004 {published data only}
-
- Elbl L, Kral Z, Smardova L, Vasova I, Tomaskova I, Jedlicka F, et al. Changes of left ventricular function accompanying the administration of BEACOPP or ABVD regimen in the treatment of Hodgkin's disease in adults [abstract] 1975. European Journal of Haematology 2004;73(Suppl 65):20‐1.
Elbl 2006 {published data only}
-
- Elbl L, Vasova I, Kral Z, Tomaskova I, Smardova L, Wagnerova B, et al. Evaluation of acute and early cardiotoxicity in survivors of Hodgkin's disease treated with ABVD or BEACOPP regimens. Journal of Chemotherapy 2006;18(2):199‐208. - PubMed
Engert 2007 {published data only}
-
- Engert A, Franklin J, Eich HT, Brillant C, Sehlen S, Cartoni C, et al. Two cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine plus extended‐field radiotherapy is superior to radiotherapy alone in early favorable Hodgkin's lymphoma: final results of the GHSG HD7 trial. Journal of Clinical Oncology 2007;25(1527‐7755 (Electronic), 23):3495‐502. - PubMed
Engert 2012 {published data only}
-
- Engert A, Haverkamp H, Kobe C, Markova J, Renner C, Ho A, et al. Reduced‐intensity chemotherapy and PET‐guided radiotherapy in patients with advanced stage Hodgkin's lymphoma (HD15 trial): a randomised, open‐label, phase 3 non‐inferiority trial. Lancet 2012;379(9828):1791‐9. - PubMed
Ferme 2002 {published data only}
-
- Ferme C, Mounier N, Divine M, Groupe d'Etudes des Lymphomes de l'Adulte. Current clinical trials for the treatment of adult advanced‐stage Hodgkin's disease: GELA experiences. Groupe d'Etudes des Lymphomes de l'Adulte. Annals of Oncology 2002;13 Suppl 1:96‐7. - PubMed
Ferme 2007 {published data only}
-
- Ferme C, Brice P, Michallet A‐S, Lederlin P, Divine M, Casasnovas O, et al. A weekly regimen with dose escalation of doxorubicin for patients with advanced Hodgkin's lymphoma: Results of a phase II study of the Groupe d'Etudes des Lymphomes de l'Adulte (GELA). Leukemia and Lymphoma 2007;48(4):691‐8. - PubMed
Franklin 1999 {published data only}
-
- Franklin J, Sieber M, Engert A, Wolf J, Tesch HR. Toxicity and feasibility of the BEACOPP regimen for advanced stage Hodgkin's disease patients older than 65 years. Annals of Oncology 1999;10(Suppl 3):157.
Franklin 2002 {published data only}
-
- Franklin J, Diehl V. Current clinical trials for the treatment of advanced‐stage Hodgkin's disease: BEACOPP. Annals of Oncology 2002;13 Suppl 1:98‐101. - PubMed
Goldstone 1998 {published data only}
-
- Goldstone AH. The case for and against high‐dose therapy with stem cell rescue for early poor prognosis Hodgkin's disease in first remission. Annals of Oncology 1998;9(Suppl 5):S83‐5. - PubMed
Hampton 2008 {published data only}
-
- Hampton T. Phase 3 trials suggest ways to improve current hematologic cancer therapies. JAMA 2008;299(5):510‐2. - PubMed
Italiano 2006 {published data only}
-
- Italiano A, Thyss A. Hodgkin's lymphoma (HL): Recent advances and prospects. Oncologie 2006;8(4):337‐43.
Johnson 2016 {published data only}
Kelly 2002 {published data only}
-
- Kelly KM, Hutchinson RJ, Sposto R, Weiner MA, Lones MA, Perkins SL, et al. Feasibility of upfront dose‐intensive chemotherapy in children with advanced‐stage Hodgkin's lymphoma: preliminary results from the Children's Cancer Group Study CCG‐59704. Annals of Oncology 2002;13 Suppl 1:107‐11. - PubMed
Koumarianou 2007 {published data only}
-
- Koumarianou AA, Xiros N, Papageorgiou E, Pectasides D, Economopoulos T. Survival improvement of young patients, aged 16‐23, with Hodgkin lymphoma (HL) during the last three decades. Anticancer Research 2007;27(2):1191‐7. - PubMed
Niitsu 2006 {published data only}
-
- Niitsu N, Okamoto M, Tomita N, Aoki S, Tamaru J, Miura I, et al. Multicentre phase II study of the baseline BEACOPP regimen for patients with advanced‐stage Hodgkin's lymphoma. Leukemia & Lymphoma 2006;47(9):1908‐14. - PubMed
Portlock 1999 {published data only}
-
- Portlock CS. Greater curability in advanced Hodgkin's disease?[comment]. Cancer Journal from Scientific American 1999;5(5):264‐5. - PubMed
Proctor 2005 {published data only}
-
- Proctor SJ, White J, Jones GL. An international approach to the treatment of Hodgkin's disease in the elderly: Launch of the SHIELD study programme. European Journal of Haematology. Supplementum 2005;75(66):63‐7. - PubMed
Sieber 1999 {published data only}
-
- Sieber M, Franklin J, Wolf J, Engert A, Paulus U, Tesch H, et al. Acute toxicity limits the feasible dose and effectiveness of BEACOPP chemotherapy in advanced stage Hodgkin's disease patients older than 65 years. Blood. 1999; Vol. 94, issue 10 Suppl 1:528a.
Sieniawski 2008 {published data only}
-
- Sieniawski M, Reineke T, Josting A, Nogova L, Behringer K, Halbsguth T, et al. Assessment of male fertility in patients with Hodgkin's lymphoma treated in the German Hodgkin Study Group (GHSG) clinical trials. Annals of Oncology 2008;19(10):1795‐801. - PubMed
Sieniawski 2008a {published data only}
-
- Sieniawski M, Reineke T, Nogova L, Josting A, Pfistner B, Diehl V, et al. Fertility in male patients with advanced Hodgkin lymphoma treated with BEACOPP: a report of the German Hodgkin Study Group (GHSG). Blood 2008;111(1):71‐6. - PubMed
Tesch 1995 {published data only}
-
- Tesch H, Lathan B, Hasenclever D, Ruffer U, Sieber M, Engert A, et al. Dose escalation for advanced stage Hodgkin's disease using the BEACOPP scheme: studies of the German Hodgkin's Study Group (GHSG). Blood. 1995; Vol. 86, issue 10 Suppl 1:439a.
Tesch 1996 {published data only}
-
- Tesch H, Lathan B, Ruffer U, Sieber M, Engert A, Franklin J, et al. Escalation of dose intensity for advanced stage Hodgkin's disease using the BEACOPP scheme ‐ studies of the German Hodgkin Study Group (GHSG). Blood 1996;88(10 Suppl Pt 1):673a, Abstract.
Tesch 1998 {published data only}
-
- Tesch H, Diehl V, Lathan B, Hasenclever D, Sieber M, Ruffer U, et al. Moderate dose escalation for advanced stage Hodgkin's disease using the bleomycin, etoposide, adriamycin, cyclophosphamide, vincristine, procarbazine, and prednisone scheme and adjuvant radiotherapy: a study of the German Hodgkin's Lymphoma Study Group. Blood 1998;92(12):4560‐7. - PubMed
Van der Kaaij 2007 {published data only}
-
- Kaaij MA, Heutte N, Stang N, Raemaekers JM, Simons AH, Carde P, et al. Gonadal function in males after chemotherapy for early‐stage Hodgkin's lymphoma treated in four subsequent trials by the European Organisation for Research and Treatment of Cancer: EORTC Lymphoma Group and the Groupe d'Etude des Lymphomes de l'Adulte. Journal of Clinical Oncology 2007;25(19):2825‐32. - PubMed
Additional references
Bonfante 1997
-
- Bonfante V, Santoro A, Viviani S, Devizzi L, Balzarotti M, Soncini F, et al. Outcome of patients with Hodgkin's disease failing after primary MOPP‐ABVD. Journal of Clinical Oncology 1997;15(0732‐183X):528‐34. - PubMed
Canellos 1992
-
- Canellos GP, Anderson JR, Propert KJ, Nissen N, Cooper MR, Henderson ES, et al. Chemotherapy of advanced Hodgkin's disease with MOPP, ABVD, or MOPP alternating with ABVD. New England Journal of Medicine 1992;327(0028‐4793 (Print), 21):1478‐84. - PubMed
Canellos 2005a
-
- Canellos GP. Clinical trials in North America. European Journal of Haematology. Supplementum. 2005, issue 0902‐4506 (Print), 66:121‐4. - PubMed
Carbone 1971
-
- Carbone PP, Kaplan HS, Musshoff K, Smithers DW, Tubiana M. Report of the Committee on Hodgkin's Disease Staging Classification. Cancer Research 1971;31(0008‐5472):1860‐1. - PubMed
Cheson 2004
-
- Cheson BD. What is new in lymphoma?. CA: A Cancer Journal for Clinicians 2004;54:260‐72. - PubMed
Cheson 2007a
-
- Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. Journal of Clinical Oncology 2007;25(1527‐7755):579‐86. - PubMed
Connors 2001
-
- Connors JM, Noordijk EM, Horning SJ. Hodgkin's lymphoma: basing the treatment on the evidence. Hematology: American Society of Hematology Education Program 2001, issue 1520‐4391:178‐93. - PubMed
Connors 2005a
-
- Connors JM. State‐of‐the‐art therapeutics: Hodgkin's lymphoma. Journal of Clinical Oncology 2005;23(0732‐183X):6400‐8. - PubMed
Deeks 2011
-
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses.. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011.
Diehl 2001
-
- Diehl V, Mauch P, Harris NL. Hodgkin's disease. Cancer: Principles and Practice of Oncology. Lippincott Williams & Wilkins, 2001:2339‐87.
Diehl 2003
-
- Diehl V, Stein H, Hummel M, Zollinger R, Connors JM. Hodgkin's lymphoma: biology and treatment strategies for primary, refractory, and relapsed disease. Hematology: American Society of Hematology Education Program 2003, issue 1520‐4391:225‐47. - PubMed
Diehl 2003a
-
- Diehl V, Franklin J, Pfreundschuh M, Lathan B, Paulus U, Hasenclever D, et al. Standard and increased‐dose BEACOPP chemotherapy compared with COPP‐ABVD for advanced Hodgkin's disease. New England Journal of Medicine 2003;348(1533‐4406):2386‐95. - PubMed
Diehl 2004
-
- Diehl V, Brillant C, Franklin J, Hermann R, Greil R, Engert A, et al. BEACOPP chemotherapy for advanced Hodgkin's disease: Results of further analyses of the HD9‐ and HD12‐ trials of the German Hodgkin Study Group (GHSG). Blood. 2004; Vol. 104, issue 11:307.
Eich 2010
-
- Eich H T, Diehl V, Gorgen H, Pabst T, Markova J, Debus J, et al. Intensified chemotherapy and dose‐reduced involved‐field radiotherapy in patients with early unfavorable Hodgkin's lymphoma: Final analysis of the German Hodgkin Study Group HD11 trial. Journal of Clinical Oncology 2010;28:4199‐206. - PubMed
Engert 2003
-
- Engert A, Schiller P, Josting A, Herrmann R, Koch P, Sieber M, et al. Involved‐field radiotherapy is equally effective and less toxic compared with extended‐field radiotherapy after four cycles of chemotherapy in patients with early‐stage unfavorable Hodgkin's lymphoma: results of the HD8 trial of the German Hodgkin's Lymphoma Study Group. Journal of Clinical Oncology 2003;21(0732‐183X):3601‐8. - PubMed
Engert 2007a
-
- Engert A, Franklin J, Eich HT, Brillant C, Sehlen S, Cartoni C, et al. Two cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine plus extended‐field radiotherapy is superior to radiotherapy alone in early favorable Hodgkin's lymphoma: final results of the GHSG HD7 trial. Journal of Clinical Oncology 2007;25(1527‐7755):3495‐502. - PubMed
Engert 2009
-
- Engert A, Diehl V, Franklin J, Lohri A, Dorken B, Ludwig WD, et al. Escalated‐dose BEACOPP in the treatment of patients with advanced‐stage Hodgkin's lymphoma: 10 years of follow‐up of the GHSG HD9 study. Journal of Clinical Oncology 2009;27:4548‐54. - PubMed
Ferme 2007a
-
- Ferme C, Eghbali H, Meerwaldt J H, Rieux C, Bosq J, Berger F, et al. Chemotherapy plus involved‐field radiation in early‐stage Hodgkin's disease. New England Journal of Medicine 2007;357:1916‐27. - PubMed
Gobbi 2005
-
- Gobbi PG, Levis A, Chisesi T, Broglia C, Vitolo U, Stelitano C, et al. ABVD versus modified stanford V versus MOPPEBVCAD with optional and limited radiotherapy in intermediate‐ and advanced‐stage Hodgkin's lymphoma: final results of a multicenter randomized trial by the Intergruppo Italiano Linfomi. Journal of Clinical Oncology 2005;23(0732‐183X (Print), 36):9198‐207. - PubMed
GRADE pro 2014 [Computer program]
-
- McMaster University. GRADEpro. McMaster University, 2015.
Hasenclever 1996
-
- Hasenclever D, Loeffler M, Diehl V. Rationale for dose escalation of first line conventional chemotherapy in advanced Hodgkin's disease. German Hodgkin's Lymphoma Study Group. Annals of Oncology 1996;7 Suppl 4(0923‐7534):95‐8. - PubMed
Hasenclever 2001
-
- Hasenclever D, Brosteanu O, Gerike T, Loeffler M. Modelling of chemotherapy: the effective dose approach. Annals of Hematology 2001;80 Suppl 3:B89‐94. - PubMed
Higgins 2011a
-
- Higgins JPT, Altman DG. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011.
Higgins 2011b
-
- Higgins JPT, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011.
Josting 2002
-
- Josting A, Franklin J, May M, Koch P, Beykirch MK, Heinz J, et al. New prognostic score based on treatment outcome of patients with relapsed Hodgkin's lymphoma registered in the database of the German Hodgkin's Lymphoma Study Group. Journal of Clinical Oncology 2002;20(0732‐183X (Print), 1):221‐30. - PubMed
Klimm 2006
-
- Klimm B, Engert A, Diehl V. First‐line treatment of Hodgkin's lymphoma. Current Hematologic Malignancy Reports 2006;1(1):51‐9. - PubMed
Lefebvre 2008
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 (updated February 2008). The Cochrane Collaboration, 2008.
Lister 1989
-
- Lister TA, Crowther D, Sutcliffe SB, Glatstein E, Canellos GP, Young RC, et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin's disease: Cotswolds meeting. Journal of Clinical Oncology 1989;7(0732‐183X (Print), 11):1630‐6. - PubMed
Loeffler 1998
-
- Loeffler M, Hasenclever D, Diehl V. Model based development of the BEACOPP regimen for advanced stage Hodgkin's disease. German Hodgkin's Lymphoma Study Group. Annals of Oncology 1998;9 Suppl 5(0923‐7534 (Print)):S73‐8. - PubMed
Moher 2009
O'Connor 2011
-
- O'Connor D, Green S, Higgins JPT. Chapter 5: Defining the review question and developing criteria for including studies. In: Higgins JPT Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011 The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Parmar 1998
-
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. - PubMed
Review Manager 5.3 [Computer program]
-
- The Cochrane Collaboration. Review Manager (RevMan) [Computer program]. The Nordic Cochrane Centre. Version 5.3. Copenhagen. The Cochrane Collaboration, 2014.
SEER 2012
-
- National Cancer Institute. Surveillance Epidemiology and End Results (SEER) Stat Fact Sheets: Hodgkin Lymphoma. http://seer.cancer.gov/statfacts/html/hodg.html 2012.
Skoetz 2013
-
- Skoetz N, Trelle S, Rancea M, Haverkamp H, Diehl V, Engert A, et al. Effect of initial treatment strategy on survival of patients with advanced‐stage Hodgkin's lymphoma: a systematic review and network meta‐analysis. Lancet Oncology 2013;14(10):943‐52. [PUBMED: 23948348] - PubMed
Sterne 2011
-
- Sterne JAC, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S, editors(s). In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011.
Swerdlow 2003
-
- Swerdlow AJ. Epidemiology of Hodgkin's disease and non‐Hodgkin's lymphoma. European Journal of Nuclear Medicine and Molecular Imaging 2003;30 Suppl 1(1619‐7070 (Print)):S3‐12. - PubMed
Thomas 2002
-
- Thomas RK, Re D, Zander T, Wolf J, Diehl V. Epidemiology and etiology of Hodgkin's lymphoma. Annals of Oncology 2002;13 Suppl 4(0923‐7534 (Print)):147‐52. - PubMed
References to other published versions of this review
Bauer 2011
-
- Bauer K, Skoetz N, Monsef I, Engert A, Brillant C. Comparison of chemotherapy including escalated BEACOPP versus chemotherapy including ABVD for patients with early unfavourable or advanced stage Hodgkin lymphoma. Cochrane Database of Systematic Reviews 2011, Issue 8. [DOI: 10.1002/14651858.CD007941.pub2] - DOI - PubMed
Brilliant 2009
-
- Brillant C, Bauer K, Herbst C, Monsef I, Skoetz N, Engert A. Comparison of chemotherapy including escalated BEACOPP versus chemotherapy including ABVD for patients with early unfavourable or advanced stage Hodgkin lymphoma. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD007941] - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

