Identification of Small Molecule Translesion Synthesis Inhibitors That Target the Rev1-CT/RIR Protein-Protein Interaction
- PMID: 28541665
- PMCID: PMC5992617
- DOI: 10.1021/acschembio.6b01144
Identification of Small Molecule Translesion Synthesis Inhibitors That Target the Rev1-CT/RIR Protein-Protein Interaction
Abstract
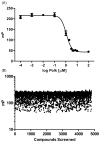
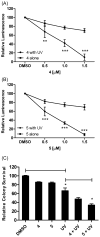
Translesion synthesis (TLS) is an important mechanism through which proliferating cells tolerate DNA damage during replication. The mutagenic Rev1/Polζ-dependent branch of TLS helps cancer cells survive first-line genotoxic chemotherapy and introduces mutations that can contribute to the acquired resistance so often observed with standard anticancer regimens. As such, inhibition of Rev1/Polζ-dependent TLS has recently emerged as a strategy to enhance the efficacy of first-line chemotherapy and reduce the acquisition of chemoresistance by decreasing tumor mutation rate. The TLS DNA polymerase Rev1 serves as an integral scaffolding protein that mediates the assembly of the active multiprotein TLS complexes. Protein-protein interactions (PPIs) between the C-terminal domain of Rev1 (Rev1-CT) and the Rev1-interacting region (RIR) of other TLS DNA polymerases play an essential role in regulating TLS activity. To probe whether disrupting the Rev1-CT/RIR PPI is a valid approach for developing a new class of targeted anticancer agents, we designed a fluorescence polarization-based assay that was utilized in a pilot screen for small molecule inhibitors of this PPI. Two small molecule scaffolds that disrupt this interaction were identified, and secondary validation assays confirmed that compound 5 binds to Rev1-CT at the RIR interface. Finally, survival and mutagenesis assays in mouse embryonic fibroblasts and human fibrosarcoma HT1080 cells treated with cisplatin and ultraviolet light indicate that these compounds inhibit mutagenic Rev1/Polζ-dependent TLS in cells, validating the Rev1-CT/RIR PPI for future anticancer drug discovery and identifying the first small molecule inhibitors of TLS that target Rev1-CT.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

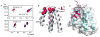
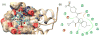


Similar articles
-
Interaction between the Rev1 C-Terminal Domain and the PolD3 Subunit of Polζ Suggests a Mechanism of Polymerase Exchange upon Rev1/Polζ-Dependent Translesion Synthesis.Biochemistry. 2016 Apr 5;55(13):2043-53. doi: 10.1021/acs.biochem.5b01282. Epub 2016 Mar 24. Biochemistry. 2016. PMID: 26982350 Free PMC article.
-
Structural Approach To Identify a Lead Scaffold That Targets the Translesion Synthesis Polymerase Rev1.J Chem Inf Model. 2018 Nov 26;58(11):2266-2277. doi: 10.1021/acs.jcim.8b00535. Epub 2018 Oct 19. J Chem Inf Model. 2018. PMID: 30289707
-
Virtual Pharmacophore Screening Identifies Small-Molecule Inhibitors of the Rev1-CT/RIR Protein-Protein Interaction.ChemMedChem. 2019 Sep 4;14(17):1610-1617. doi: 10.1002/cmdc.201900307. Epub 2019 Aug 21. ChemMedChem. 2019. PMID: 31361935 Free PMC article.
-
[Structural Basis of the Multifunctional Hub Protein and Identification of a Small-molecule Compound for Drug Discovery].Yakugaku Zasshi. 2019;139(7):969-973. doi: 10.1248/yakushi.19-00092. Yakugaku Zasshi. 2019. PMID: 31257254 Review. Japanese.
-
The Rev1-Polζ translesion synthesis mutasome: Structure, interactions and inhibition.Enzymes. 2019;45:139-181. doi: 10.1016/bs.enz.2019.07.001. Epub 2019 Aug 9. Enzymes. 2019. PMID: 31627876 Free PMC article. Review.
Cited by
-
Inhibition of the translesion synthesis polymerase REV1 exploits replication gaps as a cancer vulnerability.Sci Adv. 2020 Jun 10;6(24):eaaz7808. doi: 10.1126/sciadv.aaz7808. eCollection 2020 Jun. Sci Adv. 2020. PMID: 32577513 Free PMC article.
-
Human CST complex restricts excessive PrimPol repriming upon UV induced replication stress by suppressing p21.Nucleic Acids Res. 2024 Apr 24;52(7):3778-3793. doi: 10.1093/nar/gkae078. Nucleic Acids Res. 2024. PMID: 38348929 Free PMC article.
-
REV1: A novel biomarker and potential therapeutic target for various cancers.Front Genet. 2022 Sep 29;13:997970. doi: 10.3389/fgene.2022.997970. eCollection 2022. Front Genet. 2022. PMID: 36246647 Free PMC article.
-
Exploiting replication gaps for cancer therapy.Mol Cell. 2022 Jul 7;82(13):2363-2369. doi: 10.1016/j.molcel.2022.04.023. Epub 2022 May 13. Mol Cell. 2022. PMID: 35568026 Free PMC article. Review.
-
Inhibition of Human DNA Polymerases Eta and Kappa by Indole-Derived Molecules Occurs through Distinct Mechanisms.ACS Chem Biol. 2019 Jun 21;14(6):1337-1351. doi: 10.1021/acschembio.9b00304. Epub 2019 May 22. ACS Chem Biol. 2019. PMID: 31082191 Free PMC article.
References
-
- Chen X, Wu Y, Dong H, Zhang CY, Zhang Y. Platinum-based agents for individualized cancer treatment. Curr Mol Med. 2013;13:1603–1612. - PubMed
-
- Chen X, Wu Y, Dong H, Zhang CY, Zhang Y. Platinum-based agents for individualized cancer treatment. Curr Mol Med. 2013;13:1603–1612. - PubMed
-
- Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007;7:573–584. - PubMed
-
- Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–7279. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

