Limited engraftment of donor microbiome via one-time fecal microbial transplantation in treated HIV-infected individuals
- PMID: 28541799
- PMCID: PMC5628639
- DOI: 10.1080/19490976.2017.1334034
Limited engraftment of donor microbiome via one-time fecal microbial transplantation in treated HIV-infected individuals
Abstract
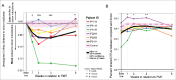
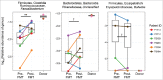
Many HIV-infected individuals on antiretroviral therapy (ART) exhibit persistent systemic inflammation, which predicts morbidity and mortality. ART-treated subjects concurrently exhibit marked compositional alterations in the gut bacterial microbiota and the degree of dysbiosis correlates with systemic inflammation. Whether interventions to modulate the microbiome can affect systemic inflammation is unknown. An open-label fecal microbial transplantation (FMT) was delivered by colonoscopy to asymptomatic HIV-infected ART-suppressed individuals without antibiotic pre-treatment. Stool was assessed before and after FMT for engraftment of donor microbes, and peripheral blood was assayed for immune activation biomarkers. Six participants received FMT and 2 participants served as controls. No serious adverse effects occurred during 24 weeks of follow-up. At baseline, HIV-infected individuals exhibited microbiota profiles distinct from uninfected donors. During the 8 weeks post-FMT, recipients demonstrated partial engraftment of the donor microbiome (P < 0.05). Recipient microbiota remained significantly distant from donors, unlike that observed following FMT for treatment of C. difficile infection. Systemic inflammatory markers showed no significant change post-FMT. FMT was well-tolerated in ART-treated, HIV-infected individuals. Engraftment was detectable but modest, and appeared to be limited to specific bacterial taxa. Whether antibiotic conditioning can enhance engraftment and the capacity of microbiota to modulate inflammation remains to be investigated.
Keywords: HIV; Microbiota; engraftment; fecal microbiome transplant; fecal transplant; inflammation; microbiome engraftment.
Figures

References
-
- Guadalupe M, Reay E, Sankaran S, Prindiville T, Flamm J, McNeil A, Dandekar S. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol 2003; 77:11708-17; PMID:14557656; https://doi.org/10.1128/JVI.77.21.11708-11717.2003 - DOI - PMC - PubMed
-
- Li Q, Duan L, Estes JD, Ma ZM, Rourke T, Wang Y, Reilly C, Carlis J, Miller CJ, Haase AT. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature 2005; 434:1148-52; PMID:15793562; https://doi.org/10.1038/nature03513 - DOI - PubMed
-
- Favre D, Mold J, Hunt PW, Kanwar B, Loke P, Seu L, Barbour JD, Lowe MM, Jayawardene A, Aweeka F, et al.. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci Transl Med 2010; 2:32ra6; PMID:20484731; https://doi.org/10.1126/scitranslmed.3000632 - DOI - PMC - PubMed
-
- Brenchley JM, Paiardini M, Knox KS, Asher AI, Cervasi B, Asher TE, Scheinberg P, Price DA, Hage CA, Kholi LM, et al.. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood 2008; 112:2826-35; PMID:18664624; https://doi.org/10.1182/blood-2008-05-159301 - DOI - PMC - PubMed
-
- Raffatellu M, Santos RL, Verhoeven DE, George MD, Wilson RP, Winter SE, Godinez I, Sankaran S, Paixao TA, Gordon MA, et al.. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med 2008; 14:421-8; PMID:18376406; https://doi.org/10.1038/nm1743 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
