Distinct responses of Purkinje neurons and roles of simple spikes during associative motor learning in larval zebrafish
- PMID: 28541889
- PMCID: PMC5444900
- DOI: 10.7554/eLife.22537
Distinct responses of Purkinje neurons and roles of simple spikes during associative motor learning in larval zebrafish
Abstract
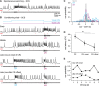
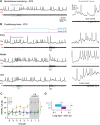
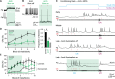
To study cerebellar activity during learning, we made whole-cell recordings from larval zebrafish Purkinje cells while monitoring fictive swimming during associative conditioning. Fish learned to swim in response to visual stimulation preceding tactile stimulation of the tail. Learning was abolished by cerebellar ablation. All Purkinje cells showed task-related activity. Based on how many complex spikes emerged during learned swimming, they were classified as multiple, single, or zero complex spike (MCS, SCS, ZCS) cells. With learning, MCS and ZCS cells developed increased climbing fiber (MCS) or parallel fiber (ZCS) input during visual stimulation; SCS cells fired complex spikes associated with learned swimming episodes. The categories correlated with location. Optogenetically suppressing simple spikes only during visual stimulation demonstrated that simple spikes are required for acquisition and early stages of expression of learned responses, but not their maintenance, consistent with a transient, instructive role for simple spikes during cerebellar learning in larval zebrafish.
Keywords: archaerhodopsin; cerebellum; classical conditioning; complex spike; neuroscience; sensorimotor integration; zebrafish.
Conflict of interest statement
IMR: Reviewing editor,
The other authors declare that no competing interests exist.
Figures






Similar articles
-
Integration of Swimming-Related Synaptic Excitation and Inhibition by olig2+ Eurydendroid Neurons in Larval Zebrafish Cerebellum.J Neurosci. 2020 Apr 8;40(15):3063-3074. doi: 10.1523/JNEUROSCI.2322-19.2020. Epub 2020 Mar 5. J Neurosci. 2020. PMID: 32139583 Free PMC article.
-
Responses of cerebellar Purkinje cells during fictive optomotor behavior in larval zebrafish.J Neurophysiol. 2016 Nov 1;116(5):2067-2080. doi: 10.1152/jn.00042.2016. Epub 2016 Aug 10. J Neurophysiol. 2016. PMID: 27512018 Free PMC article.
-
Rapid development of Purkinje cell excitability, functional cerebellar circuit, and afferent sensory input to cerebellum in zebrafish.Front Neural Circuits. 2014 Dec 19;8:147. doi: 10.3389/fncir.2014.00147. eCollection 2014. Front Neural Circuits. 2014. PMID: 25565973 Free PMC article.
-
Complex Spike Wars: a New Hope.Cerebellum. 2018 Dec;17(6):735-746. doi: 10.1007/s12311-018-0960-3. Cerebellum. 2018. PMID: 29982917 Free PMC article. Review.
-
Climbing fibers mediate vestibular modulation of both "complex" and "simple spikes" in Purkinje cells.Cerebellum. 2015 Oct;14(5):597-612. doi: 10.1007/s12311-015-0725-1. Cerebellum. 2015. PMID: 26424151 Review.
Cited by
-
Zebrafish Exploit Visual Cues and Geometric Relationships to Form a Spatial Memory.iScience. 2019 Sep 27;19:119-134. doi: 10.1016/j.isci.2019.07.013. Epub 2019 Jul 15. iScience. 2019. PMID: 31369985 Free PMC article.
-
Modeling Neurodegenerative Spinocerebellar Ataxia Type 13 in Zebrafish Using a Purkinje Neuron Specific Tunable Coexpression System.J Neurosci. 2019 May 15;39(20):3948-3969. doi: 10.1523/JNEUROSCI.1862-18.2019. Epub 2019 Mar 12. J Neurosci. 2019. PMID: 30862666 Free PMC article.
-
Development, circuitry, and function of the zebrafish cerebellum.Cell Mol Life Sci. 2023 Jul 25;80(8):227. doi: 10.1007/s00018-023-04879-5. Cell Mol Life Sci. 2023. PMID: 37490159 Free PMC article. Review.
-
Simple and complex spike responses of mouse cerebellar Purkinje neurons to regular trains and omissions of somatosensory stimuli.J Neurophysiol. 2021 Sep 1;126(3):763-776. doi: 10.1152/jn.00170.2021. Epub 2021 Aug 4. J Neurophysiol. 2021. PMID: 34346760 Free PMC article.
-
Investigation of hindbrain activity during active locomotion reveals inhibitory neurons involved in sensorimotor processing.Sci Rep. 2018 Sep 11;8(1):13615. doi: 10.1038/s41598-018-31968-4. Sci Rep. 2018. PMID: 30206288 Free PMC article.
References
-
- Amo R, Fredes F, Kinoshita M, Aoki R, Aizawa H, Agetsuma M, Aoki T, Shiraki T, Kakinuma H, Matsuda M, Yamazaki M, Takahoko M, Tsuboi T, Higashijima S, Miyasaka N, Koide T, Yabuki Y, Yoshihara Y, Fukai T, Okamoto H. The habenulo-raphe serotonergic circuit encodes an aversive expectation value essential for adaptive active avoidance of danger. Neuron. 2014;84:1034–1048. doi: 10.1016/j.neuron.2014.10.035. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

