An agent-based model of leukocyte transendothelial migration during atherogenesis
- PMID: 28542193
- PMCID: PMC5444619
- DOI: 10.1371/journal.pcbi.1005523
An agent-based model of leukocyte transendothelial migration during atherogenesis
Abstract
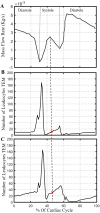
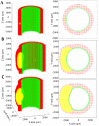
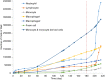
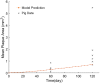
A vast amount of work has been dedicated to the effects of hemodynamics and cytokines on leukocyte adhesion and trans-endothelial migration (TEM) and subsequent accumulation of leukocyte-derived foam cells in the artery wall. However, a comprehensive mechanobiological model to capture these spatiotemporal events and predict the growth and remodeling of an atherosclerotic artery is still lacking. Here, we present a multiscale model of leukocyte TEM and plaque evolution in the left anterior descending (LAD) coronary artery. The approach integrates cellular behaviors via agent-based modeling (ABM) and hemodynamic effects via computational fluid dynamics (CFD). In this computational framework, the ABM implements the diffusion kinetics of key biological proteins, namely Low Density Lipoprotein (LDL), Tissue Necrosis Factor alpha (TNF-α), Interlukin-10 (IL-10) and Interlukin-1 beta (IL-1β), to predict chemotactic driven leukocyte migration into and within the artery wall. The ABM also considers wall shear stress (WSS) dependent leukocyte TEM and compensatory arterial remodeling obeying Glagov's phenomenon. Interestingly, using fully developed steady blood flow does not result in a representative number of leukocyte TEM as compared to pulsatile flow, whereas passing WSS at peak systole of the pulsatile flow waveform does. Moreover, using the model, we have found leukocyte TEM increases monotonically with decreases in luminal volume. At critical plaque shapes the WSS changes rapidly resulting in sudden increases in leukocyte TEM suggesting lumen volumes that will give rise to rapid plaque growth rates if left untreated. Overall this multi-scale and multi-physics approach appropriately captures and integrates the spatiotemporal events occurring at the cellular level in order to predict leukocyte transmigration and plaque evolution.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Willerson JT, Ridker PM. Inflammation as a cardiovascular risk factor. Circulation. 2004;109(21 Suppl 1):Ii2–10. Epub 2004/06/03. - PubMed
-
- Ross R. Atherosclerosis—An Inflammatory Disease. New England Journal of Medicine. 1999;340(2):115–26. doi: 10.1056/NEJM199901143400207 - DOI - PubMed
-
- Libby P. The Vascuar Biology of Atherosclerosis In: Braunwald E, Zipes DP, Libby P, editors. Heart Disease A Textbook of Cardiovascular Medicine. 6 Philadelphia: W.B. Sauders Company; 2001. p. 995–1009.
-
- Luscinskas FW, Ma S, Nusrat A, Parkos CA, Shaw SK. Leukocyte transendothelial migration: A junctional affair. Seminars in Immunology. 2002;14(2):105–13. doi: 10.1006/smim.2001.0347 - DOI - PubMed
-
- Muller WA. Mechanisms of Leukocyte Transendothelial Migration. Annual review of pathology. 2011;6:323–44. doi: 10.1146/annurev-pathol-011110-130224 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

