Measuring changes in transmission of neglected tropical diseases, malaria, and enteric pathogens from quantitative antibody levels
- PMID: 28542223
- PMCID: PMC5453600
- DOI: 10.1371/journal.pntd.0005616
Measuring changes in transmission of neglected tropical diseases, malaria, and enteric pathogens from quantitative antibody levels
Abstract
Background: Serological antibody levels are a sensitive marker of pathogen exposure, and advances in multiplex assays have created enormous potential for large-scale, integrated infectious disease surveillance. Most methods to analyze antibody measurements reduce quantitative antibody levels to seropositive and seronegative groups, but this can be difficult for many pathogens and may provide lower resolution information than quantitative levels. Analysis methods have predominantly maintained a single disease focus, yet integrated surveillance platforms would benefit from methodologies that work across diverse pathogens included in multiplex assays.
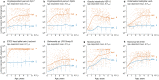
Methods/principal findings: We developed an approach to measure changes in transmission from quantitative antibody levels that can be applied to diverse pathogens of global importance. We compared age-dependent immunoglobulin G curves in repeated cross-sectional surveys between populations with differences in transmission for multiple pathogens, including: lymphatic filariasis (Wuchereria bancrofti) measured before and after mass drug administration on Mauke, Cook Islands, malaria (Plasmodium falciparum) before and after a combined insecticide and mass drug administration intervention in the Garki project, Nigeria, and enteric protozoans (Cryptosporidium parvum, Giardia intestinalis, Entamoeba histolytica), bacteria (enterotoxigenic Escherichia coli, Salmonella spp.), and viruses (norovirus groups I and II) in children living in Haiti and the USA. Age-dependent antibody curves fit with ensemble machine learning followed a characteristic shape across pathogens that aligned with predictions from basic mechanisms of humoral immunity. Differences in pathogen transmission led to shifts in fitted antibody curves that were remarkably consistent across pathogens, assays, and populations. Mean antibody levels correlated strongly with traditional measures of transmission intensity, such as the entomological inoculation rate for P. falciparum (Spearman's rho = 0.75). In both high- and low transmission settings, mean antibody curves revealed changes in population mean antibody levels that were masked by seroprevalence measures because changes took place above or below the seropositivity cutoff.
Conclusions/significance: Age-dependent antibody curves and summary means provided a robust and sensitive measure of changes in transmission, with greatest sensitivity among young children. The method generalizes to pathogens that can be measured in high-throughput, multiplex serological assays, and scales to surveillance activities that require high spatiotemporal resolution. Our results suggest quantitative antibody levels will be particularly useful to measure differences in exposure for pathogens that elicit a transient antibody response or for monitoring populations with very high- or very low transmission, when seroprevalence is less informative. The approach represents a new opportunity to conduct integrated serological surveillance for neglected tropical diseases, malaria, and other infectious diseases with well-defined antigen targets.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Hens N, Shkedy Z, Aerts M, Damme CFPV, Beutels P. Modeling Infectious Disease Parameters Based on Serological and Social Contact Data. Springer; 2012.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

