Haemocytes collected from experimentally infected Pacific oysters, Crassostrea gigas: Detection of ostreid herpesvirus 1 DNA, RNA, and proteins in relation with inhibition of apoptosis
- PMID: 28542284
- PMCID: PMC5436676
- DOI: 10.1371/journal.pone.0177448
Haemocytes collected from experimentally infected Pacific oysters, Crassostrea gigas: Detection of ostreid herpesvirus 1 DNA, RNA, and proteins in relation with inhibition of apoptosis
Abstract
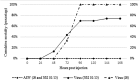
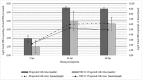
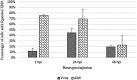
Recent transcriptomic approaches focused on anti-viral immunity in molluscs lead to the assumption that the innate immune system, such as apoptosis, plays a crucial role against ostreid herpesvirus type 1 (OsHV-1), infecting Pacific cupped oyster, Crassostrea gigas. Apoptosis constitutes a major mechanism of anti-viral response by limiting viral spread and eliminating infected cells. In this way, an OsHV-1 challenge was performed and oysters were monitored at three times post injection to investigate viral infection and host response: 2h (early after viral injection in the adductor muscle), 24h (intermediate time), and 48h (just before first oyster mortality record). Virus infection, associated with high cumulative mortality rates (74% and 100%), was demonstrated in haemocytes by combining several detection techniques such as real-time PCR, real-time RT PCR, immunofluorescence assay, and transmission electron microscopy examination. High viral DNA amounts ranged from 5.46×104 to 3.68×105 DNA copies ng-1 of total DNA, were detected in dead oysters and an increase of viral transcripts was observed from 2, 24, and 48hpi for the five targeted OsHV-1 genes encoding three putative membrane proteins (ORFs 25, 41, and 72), a putative dUTPase (ORF 75), and a putative apoptosis inhibitor (ORF 87). Apoptosis was studied at molecular and cellular levels with an early marker (phosphatidyl-serine externalisation measured by flow cytometry and epifluorescence microscopy) and a later parameter (DNA fragmentation by terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling assay (TUNEL)). The down-regulation of genes encoding proteins involved in the activation of the apoptotic pathway (TNF and caspase 3) and the up-regulation of genes encoding anti-apoptotic proteins (IAP-2, and Bcl-2) suggested an important anti-apoptosis phenomenon in haemocytes from OsHV-1 infected oysters at 24 and 48hpi. Additionally, more phosphatidyl-serines were externalized and more cells with DNA fragmentation were observed in haemocytes collected from artificial seawater injected oysters than in haemocytes collected from OsHV-1 infected oysters at 24 and 48hpi, suggesting an inhibition of the apoptotic process in presence of the virus. In conclusion, this study is the first to focus on C. gigas haemocytes, cells involved in the host immune defense, during an OsHV-1 challenge in controlled conditions by combining various and original approaches to investigate apoptosis at molecular and cellular levels.
Conflict of interest statement
Figures







Similar articles
-
Antiviral Defense and Innate Immune Memory in the Oyster.Viruses. 2018 Mar 16;10(3):133. doi: 10.3390/v10030133. Viruses. 2018. PMID: 29547519 Free PMC article. Review.
-
In situ localization and tissue distribution of ostreid herpesvirus 1 proteins in infected Pacific oyster, Crassostrea gigas.J Invertebr Pathol. 2016 May;136:124-35. doi: 10.1016/j.jip.2016.04.002. Epub 2016 Apr 8. J Invertebr Pathol. 2016. PMID: 27066775
-
In situ hybridization and histopathological observations during ostreid herpesvirus-1-associated mortalities in Pacific oysters Crassostrea gigas.Dis Aquat Organ. 2016 Nov 22;122(1):43-55. doi: 10.3354/dao03062. Dis Aquat Organ. 2016. PMID: 27901503
-
Poly I:C induces a protective antiviral immune response in the Pacific oyster (Crassostrea gigas) against subsequent challenge with Ostreid herpesvirus (OsHV-1 μvar).Fish Shellfish Immunol. 2013 Aug;35(2):382-8. doi: 10.1016/j.fsi.2013.04.051. Epub 2013 May 15. Fish Shellfish Immunol. 2013. PMID: 23685009
-
Propidium monoazide PCR, a method to determine OsHV-1 undamaged capsids and to estimate virus Lethal Dose 50.Virus Res. 2024 Feb;340:199307. doi: 10.1016/j.virusres.2023.199307. Epub 2024 Jan 4. Virus Res. 2024. PMID: 38160910 Free PMC article. Review.
Cited by
-
The Pacific Oyster Mortality Syndrome, a Polymicrobial and Multifactorial Disease: State of Knowledge and Future Directions.Front Immunol. 2021 Feb 18;12:630343. doi: 10.3389/fimmu.2021.630343. eCollection 2021. Front Immunol. 2021. PMID: 33679773 Free PMC article. Review.
-
Antiviral Defense and Innate Immune Memory in the Oyster.Viruses. 2018 Mar 16;10(3):133. doi: 10.3390/v10030133. Viruses. 2018. PMID: 29547519 Free PMC article. Review.
-
Oyster RNA-seq Data Support the Development of Malacoherpesviridae Genomics.Front Microbiol. 2017 Aug 9;8:1515. doi: 10.3389/fmicb.2017.01515. eCollection 2017. Front Microbiol. 2017. PMID: 28848525 Free PMC article.
-
Comparative Proteomics of Ostreid Herpesvirus 1 and Pacific Oyster Interactions With Two Families Exhibiting Contrasted Susceptibility to Viral Infection.Front Immunol. 2021 Jan 18;11:621994. doi: 10.3389/fimmu.2020.621994. eCollection 2020. Front Immunol. 2021. PMID: 33537036 Free PMC article.
-
Monitoring Autophagy at Cellular and Molecular Level in Crassostrea gigas During an Experimental Ostreid Herpesvirus 1 (OsHV-1) Infection.Front Cell Infect Microbiol. 2022 Apr 4;12:858311. doi: 10.3389/fcimb.2022.858311. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35444958 Free PMC article.
References
-
- Davison AJ, Eberle R, Ehlers B, Hayward GS, McGeoch DJ, Minson AC, et al. The order Herpesvirales. Arch Virol. 2009;154: 171–177. doi: 10.1007/s00705-008-0278-4 - DOI - PMC - PubMed
-
- Davison AJ, Trus BL, Cheng N, Steven AC, Watson MS, Cunningham C, et al. A novel class of herpesvirus with bivalve hosts. J Gen Virol. 2005;86: 41–53. doi: 10.1099/vir.0.80382-0 - DOI - PubMed
-
- Le Deuff RM, Renault T. Purification and partial genome characterization of a herpes-like virus infecting the Japanese oyster, Crassostrea gigas. J Gen Virol. 1999;80 (Pt 5): 1317–1322. - PubMed
-
- Martenot C, Denechère L, Hubert P, Metayer L, Oden E, Trancart S, et al. Virulence of Ostreid herpesvirus 1 μVar in sea water at 16°C and 25°C. Aquaculture. 2015;439: 1–6.
-
- Martenot C, Fourour S, Oden E, Jouaux A, Travaillé E, Malas JP, et al. Detection of the OsHV-1 μVar in the Pacific oyster Crassostrea gigas before 2008 in France and description of two new microvariants of the Ostreid Herpesvirus 1 (OsHV-1). Aquaculture. 2012;338–341: 293–296.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

