Performance evaluation of BD FACSPresto™ point of care CD4 analyzer to enumerate CD4 counts for monitoring HIV infected individuals in Nigeria
- PMID: 28542359
- PMCID: PMC5444776
- DOI: 10.1371/journal.pone.0178037
Performance evaluation of BD FACSPresto™ point of care CD4 analyzer to enumerate CD4 counts for monitoring HIV infected individuals in Nigeria
Abstract
Background: Despite the upsurge in support and intervention of donor agencies in HIV care and treatment programing in Sub-Sahara African, antiretroviral (ART) programs are still confronted with access and coverage challenges which influence enrolment of new patients. This study investigated the validity of point of care BD FACSPresto™ CD4 analyzer for CD4+ cell count, overall agreement, correlation, sensitivity, and specificity in comparison to a reference standard flow cytometry method. We also assessed the feasibility of use among non-laboratorians.
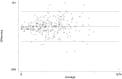
Methods: Blood samples from 300 HIV infected individuals were analyzed for CD4+ T cell and CD4%, using finger prick capillary sample from 150 PMTCT clients and 150 ART clients at Murtala Mohammed Specialist Hospital, Kano, Nigeria. Their venous samples were compared on a flow cytometry reference method using BD FACSCount CD4+ count system. The accuracy of the BD FACSPresto machine in comparison to BD FACSCount was evaluated. Statistical analysis was carried out using STATA (version 12). Bland-Altman method and correlation analysis were used to analyze agreement between both measurements. In addition, sensitivity and specificity of both measurements were determined. Statistical significance was set at p-value <0.05.
Results: The mean bias and limit of agreement for CD4+ count between BD FACSPresto and BD FACS count machine were 7.49 (95% CI: 2.44 to 12.54) and -8.14 to 96.39 respectively. Further analysis revealed close agreement between BD FACSPresto and BD FACSCount with no significant difference between the two methods (p = .0.95). Using a threshold of 500 cells/μL, sensitivity and specificity of BD FACSPresto were 95.1% and 97.1% respectively, compared to BD FACSCount. There was no statistically significant difference in the misclassification between BD FACSPresto and BD FACSCount results (p = 0.23). Furthermore, sensitivity and specificity were similar when BD FACSPresto machine was operated by a nurse or laboratory scientist, there was no substantial difference in testing variability observed between laboratory and non-laboratory operators using the BD FACSPresto analyzer.
Conclusions: Overall, BD FACSPresto Point of Care CD4+ count finger stick capillary blood results is a reliable method in comparison to venous sample cytometry method and no significant difference variability observed between laboratory personnel and non-laboratory operators. The BD FACSPresto is simple, more robust and easy to use equipment without significant variability in reliability by non-laboratory health care workers hence will be a valuable instrument in increasing access and coverage of CD4 estimations in developing countries. The introduction of the BD FACSPresto POC analyzer has a high potential in reducing patients waiting time and improving the overall quality of ART service and clients' satisfaction especially in rural settings.
Conflict of interest statement
Figures
Similar articles
-
Performance of the BD-FACS Presto for CD4 count and hemoglobin measurement in a district hospital and rural laboratory in Ghana.PLoS One. 2019 Feb 22;14(2):e0212684. doi: 10.1371/journal.pone.0212684. eCollection 2019. PLoS One. 2019. PMID: 30794637 Free PMC article. Clinical Trial.
-
Performance of the BD FACSPresto near to patient analyzer in comparison with representative conventional CD4 instruments in Cameroon.AIDS Res Ther. 2020 Aug 17;17(1):53. doi: 10.1186/s12981-020-00309-9. AIDS Res Ther. 2020. PMID: 32799909 Free PMC article.
-
The BD FACSPresto Point of Care CD4 Test Accurately Enumerates CD4+ T Cell Counts.PLoS One. 2015 Dec 31;10(12):e0145586. doi: 10.1371/journal.pone.0145586. eCollection 2015. PLoS One. 2015. PMID: 26720601 Free PMC article.
-
Evaluation of a flow cytometry method for CD4 T cell enumeration based on volumetric primary CD4 gating using thermoresistant reagents.J Immunol Methods. 2011 Sep 30;372(1-2):7-13. doi: 10.1016/j.jim.2011.07.012. Epub 2011 Jul 29. J Immunol Methods. 2011. PMID: 21835181 Review.
-
Absolute and percent CD4+ T-cell enumeration by flow cytometry using capillary blood.J Immunol Methods. 2011 Sep 30;372(1-2):1-6. doi: 10.1016/j.jim.2011.07.008. Epub 2011 Jul 20. J Immunol Methods. 2011. PMID: 21787779 Review.
Cited by
-
Peripheral blood lymphocyte immunophenotyping (TBNK) - a comparison of BD FACSCanto II and BD FACSLyric flow cytometry analysers.Cent Eur J Immunol. 2024;49(1):45-51. doi: 10.5114/ceji.2024.135939. Epub 2024 Mar 5. Cent Eur J Immunol. 2024. PMID: 38812607 Free PMC article.
-
Performance of the BD-FACS Presto for CD4 count and hemoglobin measurement in a district hospital and rural laboratory in Ghana.PLoS One. 2019 Feb 22;14(2):e0212684. doi: 10.1371/journal.pone.0212684. eCollection 2019. PLoS One. 2019. PMID: 30794637 Free PMC article. Clinical Trial.
-
Validation of the BD FACSPresto system for the measurement of CD4 T-lymphocytes and hemoglobin concentration in HIV-negative and HIV-positive subjects.Sci Rep. 2020 Nov 11;10(1):19605. doi: 10.1038/s41598-020-76549-6. Sci Rep. 2020. PMID: 33177659 Free PMC article.
-
Comparison of Standard and Point-of-Care CD4+ T Lymphocyte Measurement Methods in HIV-1 Infected Turkish Patients.Medicina (Kaunas). 2024 Dec 21;60(12):2094. doi: 10.3390/medicina60122094. Medicina (Kaunas). 2024. PMID: 39768973 Free PMC article.
-
Performance of non-laboratory staff for diagnostic testing and specimen collection in HIV programs: A systematic review and meta-analysis.PLoS One. 2019 May 2;14(5):e0216277. doi: 10.1371/journal.pone.0216277. eCollection 2019. PLoS One. 2019. PMID: 31048881 Free PMC article.
References
-
- World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Geneva: World Health Organization, 2016. - PubMed
-
- Federal Ministry of Health. National Guidelines for HIV Prevention Treatment and Care. Abuja: National AIDS and STI Control Programme, Federal Ministry of Health, Nigeria, 2016.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials