Evidence for pleural epithelial-mesenchymal transition in murine compensatory lung growth
- PMID: 28542402
- PMCID: PMC5438137
- DOI: 10.1371/journal.pone.0177921
Evidence for pleural epithelial-mesenchymal transition in murine compensatory lung growth
Abstract
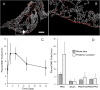
In many mammals, including rodents and humans, removal of one lung results in the compensatory growth of the remaining lung; however, the mechanism of compensatory lung growth is unknown. Here, we investigated the changes in morphology and phenotype of pleural cells after pneumonectomy. Between days 1 and 3 after pneumonectomy, cells expressing α-smooth muscle actin (SMA), a cytoplasmic marker of myofibroblasts, were significantly increased in the pleura compared to surgical controls (p < .01). Scanning electron microscopy of the pleural surface 3 days post-pneumonectomy demonstrated regions of the pleura with morphologic features consistent with epithelial-mesenchymal transition (EMT); namely, cells with disrupted intercellular junctions and an acquired mesenchymal (rounded and fusiform) morphotype. To detect the migration of the transitional pleural cells into the lung, a biotin tracer was used to label the pleural mesothelial cells at the time of surgery. By post-operative day 3, image cytometry of post-pneumonectomy subpleural alveoli demonstrated a 40-fold increase in biotin+ cells relative to pneumonectomy-plus-plombage controls (p < .01). Suggesting a similar origin in space and time, the distribution of cells expressing biotin, SMA, or vimentin demonstrated a strong spatial autocorrelation in the subpleural lung (p < .001). We conclude that post-pneumonectomy compensatory lung growth involves EMT with the migration of transitional mesothelial cells into subpleural alveoli.
Conflict of interest statement
Figures






References
-
- Hsia CCW, Berberich MA, Driscoll B, Laubach VE, Lillehei CW, Massaro C, et al. (2004) Mechanisms and limits of induced postnatal lung growth. Am J Respir Crit Care Med 170: 319–343. doi: 10.1164/rccm.200209-1062ST - DOI - PubMed
-
- Butler J, Loring SH, Patz S, Tsuda A, Yablonskiy DA, Mentzer SJ. (2012) Evidence for adult lung growth in humans. N Engl J Med 367: 244–247. doi: 10.1056/NEJMoa1203983 - DOI - PMC - PubMed
-
- Konerding MA, Gibney BC, Houdek J, Chamoto K, Ackermann M, Lee G, et al. (2012) Spatial dependence of alveolar angiogenesis in post-pneumonectomy lung growth. Angiogenesis 15: 23–32. doi: 10.1007/s10456-011-9236-y - DOI - PMC - PubMed
-
- Fehrenbach H, Voswinickel R, Michl V, Mehling T, Fehrenbach A, Seeger W, et al. (2008) Neoalveolarisation contributes to compensatory lung growth following pneumonectomy in mice. Eur Respir J 31: 515–522. doi: 10.1183/09031936.00109407 - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

