The intestinal TORC2 signaling pathway contributes to associative learning in Caenorhabditis elegans
- PMID: 28542414
- PMCID: PMC5444632
- DOI: 10.1371/journal.pone.0177900
The intestinal TORC2 signaling pathway contributes to associative learning in Caenorhabditis elegans
Abstract
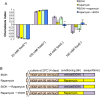
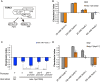
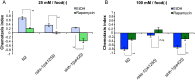
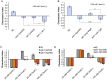
Several types of associative learning are dependent upon the presence or absence of food, and are crucial for the survival of most animals. Target of rapamycin (TOR), a kinase which exists as a component of two complexes, TOR complex 1 (TORC1) and TOR complex 2 (TORC2), is known to act as a nutrient sensor in numerous organisms. However, the in vivo roles of TOR signaling in the nervous system remain largely unclear, partly because its multifunctionality and requirement for survival make it difficult to investigate. Here, using pharmacological inhibitors and genetic analyses, we show that TORC1 and TORC2 contribute to associative learning between salt and food availability in the nematode Caenorhabditis elegans in a process called taste associative learning. Worms migrate to salt concentrations experienced previously during feeding, but they avoid salt concentrations experienced under starvation conditions. Administration of the TOR inhibitor rapamycin causes a behavioral defect after starvation conditioning. Worms lacking either RICT-1 or SINH-1, two TORC2 components, show defects in migration to high salt levels after learning under both fed and starved conditions. We also analyzed the behavioral phenotypes of mutants of the putative TORC1 substrate RSKS-1 (the C. elegans homolog of the mammalian S6 kinase S6K) and the putative TORC2 substrates SGK-1 and PKC-2 (homologs of the serum and glucocorticoid-induced kinase 1, SGK1, and protein kinase C-α, PKC-α, respectively) and found that neuronal RSKS-1 and PKC-2, as well as intestinal SGK-1, are involved in taste associative learning. Our findings shed light on the functions of TOR signaling in behavioral plasticity and provide insight into the mechanisms by which information sensed in the intestine affects the nervous system to modulate food-searching behaviors.
Conflict of interest statement
Figures

Similar articles
-
A non-canonical role for the C. elegans dosage compensation complex in growth and metabolic regulation downstream of TOR complex 2.Development. 2013 Sep;140(17):3601-12. doi: 10.1242/dev.094292. Epub 2013 Jul 24. Development. 2013. PMID: 23884442 Free PMC article.
-
TORC2 signaling antagonizes SKN-1 to induce C. elegans mesendodermal embryonic development.Dev Biol. 2013 Dec 15;384(2):214-27. doi: 10.1016/j.ydbio.2013.08.011. Epub 2013 Aug 20. Dev Biol. 2013. PMID: 23973804 Free PMC article.
-
Rictor/TORC2 regulates Caenorhabditis elegans fat storage, body size, and development through sgk-1.PLoS Biol. 2009 Mar 3;7(3):e60. doi: 10.1371/journal.pbio.1000060. PLoS Biol. 2009. PMID: 19260765 Free PMC article.
-
TORC2 Structure and Function.Trends Biochem Sci. 2016 Jun;41(6):532-545. doi: 10.1016/j.tibs.2016.04.001. Epub 2016 May 5. Trends Biochem Sci. 2016. PMID: 27161823 Review.
-
Who does TORC2 talk to?Biochem J. 2018 May 24;475(10):1721-1738. doi: 10.1042/BCJ20180130. Biochem J. 2018. PMID: 29794170 Review.
Cited by
-
The complex genetics and biology of human temperament: a review of traditional concepts in relation to new molecular findings.Transl Psychiatry. 2019 Nov 11;9(1):290. doi: 10.1038/s41398-019-0621-4. Transl Psychiatry. 2019. PMID: 31712636 Free PMC article. Review.
-
Metabolic plasticity sustains the robustness of Caenorhabditis elegans embryogenesis.EMBO Rep. 2023 Dec 6;24(12):e57440. doi: 10.15252/embr.202357440. Epub 2023 Oct 27. EMBO Rep. 2023. PMID: 37885348 Free PMC article.
-
Food-Dependent Plasticity in Caenorhabditis elegans Stress-Induced Sleep Is Mediated by TOR-FOXA and TGF-β Signaling.Genetics. 2018 Aug;209(4):1183-1195. doi: 10.1534/genetics.118.301204. Epub 2018 Jun 20. Genetics. 2018. PMID: 29925566 Free PMC article.
-
TOR Signaling in Caenorhabditis elegans Development, Metabolism, and Aging.Genetics. 2019 Oct;213(2):329-360. doi: 10.1534/genetics.119.302504. Genetics. 2019. PMID: 31594908 Free PMC article. Review.
-
The Intestine as a Lifespan- and Proteostasis-Promoting Signaling Tissue.Front Aging. 2022 Jun 2;3:897741. doi: 10.3389/fragi.2022.897741. eCollection 2022. Front Aging. 2022. PMID: 35821863 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

