ROS regulation of axonal mitochondrial transport is mediated by Ca2+ and JNK in Drosophila
- PMID: 28542430
- PMCID: PMC5436889
- DOI: 10.1371/journal.pone.0178105
ROS regulation of axonal mitochondrial transport is mediated by Ca2+ and JNK in Drosophila
Abstract
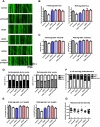
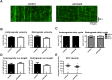
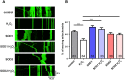
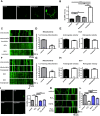
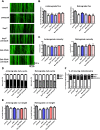
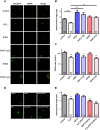
Mitochondria perform critical functions including aerobic ATP production and calcium (Ca2+) homeostasis, but are also a major source of reactive oxygen species (ROS) production. To maintain cellular function and survival in neurons, mitochondria are transported along axons, and accumulate in regions with high demand for their functions. Oxidative stress and abnormal mitochondrial axonal transport are associated with neurodegenerative disorders. However, we know little about the connection between these two. Using the Drosophila third instar larval nervous system as the in vivo model, we found that ROS inhibited mitochondrial axonal transport more specifically, primarily due to reduced flux and velocity, but did not affect transport of other organelles. To understand the mechanisms underlying these effects, we examined Ca2+ levels and the JNK (c-Jun N-terminal Kinase) pathway, which have been shown to regulate mitochondrial transport and general fast axonal transport, respectively. We found that elevated ROS increased Ca2+ levels, and that experimental reduction of Ca2+ to physiological levels rescued ROS-induced defects in mitochondrial transport in primary neuron cell cultures. In addition, in vivo activation of the JNK pathway reduced mitochondrial flux and velocities, while JNK knockdown partially rescued ROS-induced defects in the anterograde direction. We conclude that ROS have the capacity to regulate mitochondrial traffic, and that Ca2+ and JNK signaling play roles in mediating these effects. In addition to transport defects, ROS produces imbalances in mitochondrial fission-fusion and metabolic state, indicating that mitochondrial transport, fission-fusion steady state, and metabolic state are closely interrelated in the response to ROS.
Conflict of interest statement
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

