Interleukin-6 stimulates Akt and p38 MAPK phosphorylation and fibroblast migration in non-diabetic but not diabetic mice
- PMID: 28542434
- PMCID: PMC5441644
- DOI: 10.1371/journal.pone.0178232
Interleukin-6 stimulates Akt and p38 MAPK phosphorylation and fibroblast migration in non-diabetic but not diabetic mice
Abstract
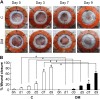
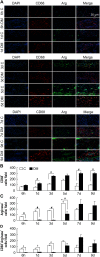
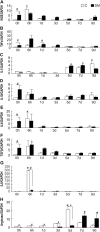
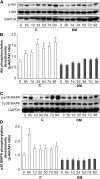
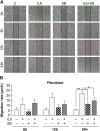
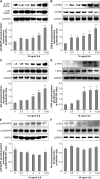
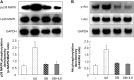
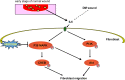
Persistent inflammatory environment and abnormal macrophage activation are characteristics of chronic diabetic wounds. Here, we attempted to characterize the differences in macrophage activation and temporal variations in cytokine expression in diabetic and non-diabetic wounds, with a focus on interleukin (IL)-6 mRNA expression and the p38 MAPK and PI3K/Akt signaling pathways. Cutaneous wound closure, CD68- and arginase-1 (Arg-1)-expressing macrophages, and cytokine mRNA expression were examined in non-diabetic and streptozotocin-induced type 1 diabetic mice at different time points after injury. The effect of IL-6 on p38 MAPK and Akt phosphorylation was investigated, and an in vitro scratch assay was performed to determine the role of IL-6 in primary skin fibroblast migration. Before injury, mRNA expression levels of the inflammatory markers iNOS, IL-6, and TNF-α were higher in diabetic mice; however, IL-6 expression was significantly lower 6 h post injury in diabetic wounds than that in non-diabetic wounds. Non-diabetic wounds exhibited increased p38 MAPK and Akt phosphorylation; however, no such increase was found in diabetic wounds. In fibroblasts from non-diabetic mice, IL-6 increased the phosphorylation of p38 MAPK and levels of its downstream factor CREB, and also significantly increased Akt phosphorylation and levels of its upstream factor P13K. These effects of IL-6 were not detected in fibroblasts derived from the diabetic mice. In scratch assays, IL-6 stimulated the migration of primary cultured skin fibroblasts from the non-diabetic mice, and the inhibition of p38 MAPK was found to markedly suppress IL-6-stimulated fibroblast migration. These findings underscore the critical differences between diabetic and non-diabetic wounds in terms of macrophage activation, cytokine mRNA expression profile, and involvement of the IL-6-stimulated p38 MAPK-Akt signaling pathway. Aberrant macrophage activation and abnormalities in the cytokine mRNA expression profile during different phases of wound healing should be addressed when designing effective therapeutic modalities for refractory diabetic wounds.
Conflict of interest statement
Figures
Similar articles
-
Impaired wound healing results from the dysfunction of the Akt/mTOR pathway in diabetic rats.J Dermatol Sci. 2015 Sep;79(3):241-51. doi: 10.1016/j.jdermsci.2015.06.002. Epub 2015 Jun 10. J Dermatol Sci. 2015. PMID: 26091964
-
Ozone oil promotes wound healing by increasing the migration of fibroblasts via PI3K/Akt/mTOR signaling pathway.Biosci Rep. 2017 Nov 9;37(6):BSR20170658. doi: 10.1042/BSR20170658. Print 2017 Dec 22. Biosci Rep. 2017. PMID: 28864782 Free PMC article.
-
Insulin promotes macrophage phenotype transition through PI3K/Akt and PPAR-γ signaling during diabetic wound healing.J Cell Physiol. 2019 Apr;234(4):4217-4231. doi: 10.1002/jcp.27185. Epub 2018 Aug 21. J Cell Physiol. 2019. PMID: 30132863
-
Mechanism of Proliferation of Cultured Human Corneal Endothelial Cells.Cornea. 2017 Nov;36 Suppl 1:S41-S45. doi: 10.1097/ICO.0000000000001337. Cornea. 2017. PMID: 28902018 Review.
-
Cutaneous Wound Healing and the Effects of Cannabidiol.Int J Mol Sci. 2024 Jun 28;25(13):7137. doi: 10.3390/ijms25137137. Int J Mol Sci. 2024. PMID: 39000244 Free PMC article. Review.
Cited by
-
Aurka deficiency in the intestinal epithelium promotes age-induced obesity via propionate-mediated AKT activation.Int J Biol Sci. 2021 Mar 25;17(5):1302-1314. doi: 10.7150/ijbs.56477. eCollection 2021. Int J Biol Sci. 2021. PMID: 33867847 Free PMC article.
-
The impact of interleukin-6 (IL-6) and mesenchymal stem cell-derived IL-6 on neurological conditions.Front Immunol. 2024 Jun 24;15:1400533. doi: 10.3389/fimmu.2024.1400533. eCollection 2024. Front Immunol. 2024. PMID: 39015561 Free PMC article. Review.
-
Infection by High-Risk Human Papillomaviruses, Epithelial-to-Mesenchymal Transition and Squamous Pre-Malignant or Malignant Lesions of the Uterine Cervix: A Series of Chained Events?Int J Mol Sci. 2021 Dec 17;22(24):13543. doi: 10.3390/ijms222413543. Int J Mol Sci. 2021. PMID: 34948338 Free PMC article. Review.
-
Specialized Pro-Resolving Mediators Do Not Inhibit the Synthesis of Inflammatory Mediators Induced by Tumor Necrosis Factor-α in Synovial Fibroblasts.Yonago Acta Med. 2022 Apr 16;65(2):111-125. doi: 10.33160/yam.2022.05.001. eCollection 2022 May. Yonago Acta Med. 2022. PMID: 35611061 Free PMC article.
-
A p38 MAPK-ROS axis fuels proliferation stress and DNA damage during CRISPR-Cas9 gene editing in hematopoietic stem and progenitor cells.Cell Rep Med. 2024 Nov 19;5(11):101823. doi: 10.1016/j.xcrm.2024.101823. Epub 2024 Nov 12. Cell Rep Med. 2024. PMID: 39536752 Free PMC article.
References
-
- Rathmann W, Giani G. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(10): 2568–2569;. - PubMed
-
- Ramsey SD, Newton K, Blough D, McCulloch DK, Sandhu N, Reiber GE, et al. Incidence, outcomes, and cost of foot ulcers in patients with diabetes. Diabetes Care. 1999;22(3): 382–387. - PubMed
-
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12): 958–969. Epub 2008/11/26. PubMed Central PMCID: PMC2724991. doi: 10.1038/nri2448 - DOI - PMC - PubMed
-
- Mangino G, Percario ZA, Fiorucci G, Vaccari G, Manrique S, Romeo G, et al. In vitro treatment of human monocytes/macrophages with myristoylated recombinant Nef of human immunodeficiency virus type 1 leads to the activation of mitogen-activated protein kinases, IkappaB kinases, and interferon regulatory factor 3 and to the release of beta interferon. J Virol. 2007;81(6): 2777–27791. PubMed Central PMCID: PMCPMC1865981. doi: 10.1128/JVI.01640-06 - DOI - PMC - PubMed
-
- Verreck FAW, de Boer T, Langenberg DML, Hoeve MA, Kramer M, Vaisberg E, et al. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc Natl Acad Sci U S A. 2004;101(13): 4560–4565. PubMed Central PMCID: PMCPMC384786. doi: 10.1073/pnas.0400983101 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

