DOT or SAT for Rifampicin-resistant tuberculosis? A non-randomized comparison in a high HIV-prevalence setting
- PMID: 28542441
- PMCID: PMC5436852
- DOI: 10.1371/journal.pone.0178054
DOT or SAT for Rifampicin-resistant tuberculosis? A non-randomized comparison in a high HIV-prevalence setting
Abstract
Background: Daily directly-observed therapy (DOT) is recommended for rifampicin-resistant tuberculosis (RR-TB) patients throughout treatment. We assessed the impact of self-administered treatment (SAT) in a South African township with high rates of RR-TB and HIV.
Methods: Community-supported SAT for patients who completed the intensive phase was piloted in five primary care clinics in Khayelitsha. We compared final treatment outcomes among RR-TB patients initiating treatment before (standard-of-care (SOC)-cohort, January 2010-July 2013) and after the implementation of the pilot (SAT-cohort, January 2012-December 2014). All patients with outcomes before January 1, 2017 were considered in the analysis of outcomes.
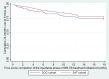
Results: One-hundred-eighteen patients in the SOC-cohort and 174 patients in the SAT-cohort had final RR-TB treatment outcomes; 70% and 73% were HIV-co-infected, respectively. The proportion of patients with a final outcome of loss to follow-up (LTFU) did not differ whether treated in the SOC (25/118, 21.2%) or SAT-cohort (31/174, 17.8%) (P = 0.47). There were no significant differences in the time to 24-month LTFU among HIV-infected and uninfected patients (HR 0.90, 95% CI: 0.51-1.6, P = 0.71), or among patients enrolled in the SOC-cohort versus the SAT-cohort (HR 0.83, 95% CI: 0.49-1.4, P = 0.50) who received at least 6-months of RR-TB treatment.
Conclusion: The introduction of SAT during the continuation phase of RR-TB treatment does not adversely affect final RR-TB treatment outcomes in a high TB and HIV-burden setting. This differentiated, patient-centred model of care could be considered in RR-TB programmes to decrease the burden of DOT on patients and health facilities.
Conflict of interest statement
Figures
Similar articles
-
Adherence interventions and outcomes of tuberculosis treatment: A systematic review and meta-analysis of trials and observational studies.PLoS Med. 2018 Jul 3;15(7):e1002595. doi: 10.1371/journal.pmed.1002595. eCollection 2018 Jul. PLoS Med. 2018. PMID: 29969463 Free PMC article.
-
Home-Based and Facility-Based Directly Observed Therapy of Tuberculosis Treatment under Programmatic Conditions in Urban Tanzania.PLoS One. 2016 Aug 11;11(8):e0161171. doi: 10.1371/journal.pone.0161171. eCollection 2016. PLoS One. 2016. PMID: 27513331 Free PMC article.
-
Severe adverse events during second-line tuberculosis treatment in the context of high HIV Co-infection in South Africa: a retrospective cohort study.BMC Infect Dis. 2016 Oct 21;16(1):593. doi: 10.1186/s12879-016-1933-0. BMC Infect Dis. 2016. PMID: 27769174 Free PMC article.
-
Twice-weekly, directly observed treatment for HIV-infected and uninfected tuberculosis patients: cohort study in rural South Africa.AIDS. 1999 May 7;13(7):811-7. doi: 10.1097/00002030-199905070-00010. AIDS. 1999. PMID: 10357380
-
HIV infection and multidrug-resistant tuberculosis: the perfect storm.J Infect Dis. 2007 Aug 15;196 Suppl 1:S86-107. doi: 10.1086/518665. J Infect Dis. 2007. PMID: 17624830 Review.
Cited by
-
Understanding the role of video direct observed therapy for patients on an oral short-course regimen for multi-drug resistant tuberculosis: findings from a qualitative study in Eswatini.BMC Infect Dis. 2024 Aug 15;24(1):829. doi: 10.1186/s12879-024-09744-9. BMC Infect Dis. 2024. PMID: 39148081 Free PMC article.
-
Community-supported self-administered tuberculosis treatment combined with active tuberculosis screening: a pilot experience in Conakry, Guinea.Glob Health Action. 2023 Dec 31;16(1):2262134. doi: 10.1080/16549716.2023.2262134. Epub 2023 Oct 6. Glob Health Action. 2023. PMID: 37799061 Free PMC article.
-
Readability of Patient-Facing Information of Antibiotics Used in the WHO Short 6-Month and 9-Month All Oral Treatment for Drug-Resistant Tuberculosis.Lung. 2024 Oct;202(5):741-751. doi: 10.1007/s00408-024-00732-z. Epub 2024 Jul 26. Lung. 2024. PMID: 39060416 Free PMC article.
-
Navigating DR-TB Treatment care: a qualitative exploration of barriers and facilitators to retention in care among people with history of early disengagement from drug-resistant tuberculosis treatment in Johannesburg, South Africa.BMC Health Serv Res. 2025 Jan 22;25(1):122. doi: 10.1186/s12913-025-12265-z. BMC Health Serv Res. 2025. PMID: 39844137 Free PMC article.
-
Adherence interventions and outcomes of tuberculosis treatment: A systematic review and meta-analysis of trials and observational studies.PLoS Med. 2018 Jul 3;15(7):e1002595. doi: 10.1371/journal.pmed.1002595. eCollection 2018 Jul. PLoS Med. 2018. PMID: 29969463 Free PMC article.
References
-
- World Health Organization. Global Tuberculosis Report. 2015.
-
- Korenromp EL, Bierrenbach AL, Williams BG, Dye C. The measurement and estimation of tuberculosis mortality. IJTLD. 2009;13: 283–303. - PubMed
-
- Shean K, Streicher E, Pieterson E, Symons G, van Zyl Smit R, Theron G, et al. Drug-associated adverse events and their relationship with outcomes in patients receiving treatment for extensively drug-resistant tuberculosis in South Africa. PLoS One. 2013;8: e63057 doi: 10.1371/journal.pone.0063057 - DOI - PMC - PubMed
-
- World Health Organization. Companion handbook to the WHO guidelines for the programmatic management of drug-resistant tuberculosis. 2014. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

